Furans are five-membered heterocycles with one oxygen atom that are commonly found, e.g., in functional materials or pharmaceutically active compounds. There are several methods for the construction of furan derivatives, which often require the prefunctionalization of substrates. The [3+2] annulation of unsaturated hydrocarbons with β-dicarbonyl compounds can be an alternative that uses readily available starting materials. However, there can still be drawbacks such as a need for strong oxidants or narrow substrate scopes.
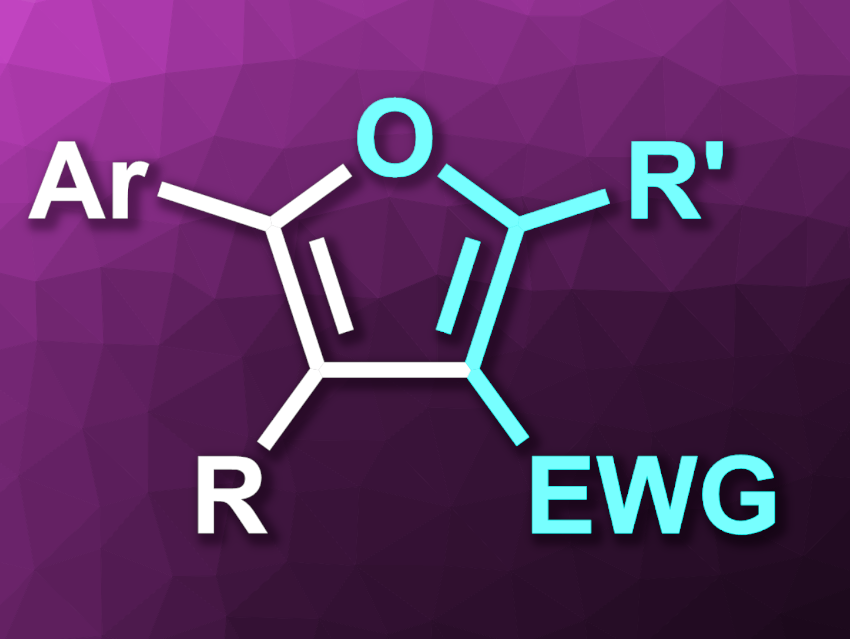
Ming Chen, Ting Li, Xinxin Zhao, Nanyang Normal University, Henan, China, and colleagues have developed an electrochemical strategy for the synthesis of tri- or tetra-substituted furan derivatives (general structure pictured) via a [3+2] annulation of alkynes and β-keto compounds, using ferrocene as a catalyst. The team used a graphite felt anode and a stainless steel cathode. The reactions were performed in MeCN at room temperature under a nitrogen atmosphere, with 5 mol% of the ferrocene catalyst, NaOAc as a base, and nBu4NClO4 as an electrolyte.
This method features mild conditions, high atom economy, and good tolerance for various alkynes and β-keto compounds. Ethynylbenzenes, internal alkynes, and even complex, drug-like molecules with alkyne groups were suitable reaction partners. Cyclic, linear, or branched diketones, as well as malonate, could be used as β-keto compounds. β-Ketoesters, β-cyanoketones, β-cyanoesters, β-ketoamides, and β-ketophosphates were also transformed successfully. The team performed a reaction on the gram scale and achieved an isolated yield of 63 %. They propose that the reaction involves a tandem radical addition and cyclization process.
- Electrocatalytic [3 + 2] Annulation for the Synthesis of Polysubstituted Furans,
Ming Chen, Jian Wang, Yuanmeng Kan, Xiaoni Jia, Binbin Huang, Ting Li, Xinxin Zhao,
Org. Lett. 2023.
https://doi.org/10.1021/acs.orglett.3c01582