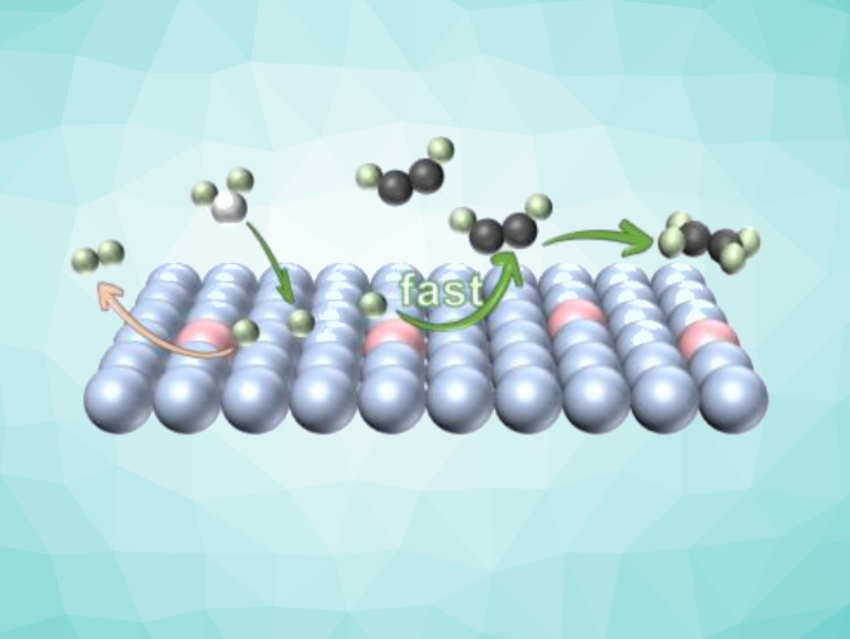
Ethylene is an important feedstock for the chemical industry. Traditionally, the production of ethylene relies on the high-temperature cracking of petroleum. The semi-hydrogenation of coal-derived acetylene to ethylene could serve as a petroleum-independent strategy. This acetylene semi-hydrogenation (EASH) could be driven by renewable energy, directly utilize water as a hydrogen source, and operate under a mild conditions. However, the process requires suitable catalysts. Available catalysts can suffer from high overpotentials and a competitive hydrogen evolution reaction (HER), which reduces the efficiency and hampers practical use.
Yifu Yu, Tianjin University, China, and colleagues have developed zinc-doped copper catalysts for acetylene semi-hydrogenation with high energy efficiency. The team prepared a series of copper-zinc alloys using an electroreduction strategy and screened them for their catalytic performance. They found that a catalyst with a Zn doping amount of 2.7 wt% performed the best.
The optimized catalyst achieved an ethylene partial current density of −0.29 A cm−2 with a Faradaic efficiency of 96 %. According to the researchers, the zinc doping significantly promotes acetylene adsorption and activation, accelerates the reaction kinetics, and effectively suppresses hydrogen evolution and over-hydrogenation. Overall, the work provides a highly efficient strategy with the potential for sustainable ethylene production.
- Electrocatalytic Acetylene Semi‐Hydrogenation to Ethylene with High Energy Efficiency,
Cong Dou, Yanmei Huang, Bohang Zhao, Weiwei Lei, Bin Zhang, Yifu Yu,
Angew. Chem. Int. Ed. 2025.
https://doi.org/10.1002/anie.202423381