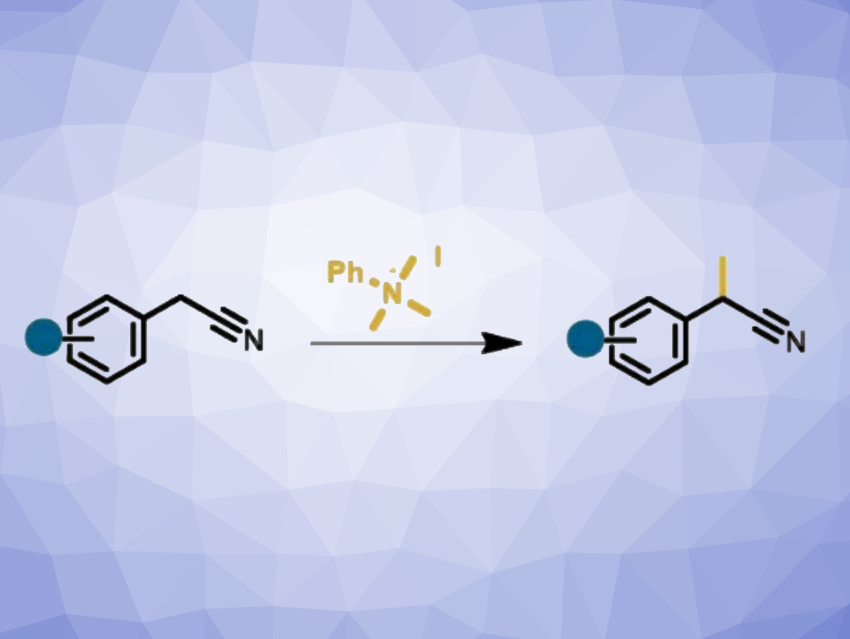
Aryl acetonitriles are useful compounds, e.g., as important precursors in the manufacturing of pharmaceutically active species. The selective monomethylation of aryl acetonitriles gives α-methylphenylacetonitriles, or 2-arylproprionitriles, which are common building blocks, e.g., for the construction of anti-inflammatory drugs such as ibuprofen or naproxen. However, achieving selective monomethylations can be challenging, and commonly used methylating agents are often hazardous and highly toxic.
Michael Schnürch, Vienna University of Technology (TU Wien), Austria, and colleagues have developed a selective and efficient metal-free protocol for the α-methylation of aryl acetonitriles to give 2-arylproprionitriles using quaternary ammonium salts. The team reacted a variety of (hetero)aryl acetonitriles, functionalized with, e.g., halides or ethers, with the quaternary ammonium salt PhMe3NI in the presence of Cs2CO3 as a base in toluene as the solvent at 120 °C.
Under these conditions, the desired methylated products were obtained in moderate to good yields, together with stochiometric amounts of N,N-dimethylaniline, which could be easily removed as its water-soluble HCl salt after an acidic workup. By adjusting the reaction conditions and using NaOtBu as a base, dimethylated biphenyl products were also obtained selectively. Overall, the method uses a safe, easy-to-handle, solid methylating agent and could be useful for the synthesis of active pharmaceutical ingredients.
- Efficient synthesis of 2‐arylpropionitriles via selective monomethylation of aryl acetonitriles using an easy to handle methylation agent,
Eleni Papaplioura, Johanna Templ, Nina Wildhack, Michael Schnürch,
Eur. J. Org. Chem. 2024.
https://doi.org/10.1002/ejoc.202400693