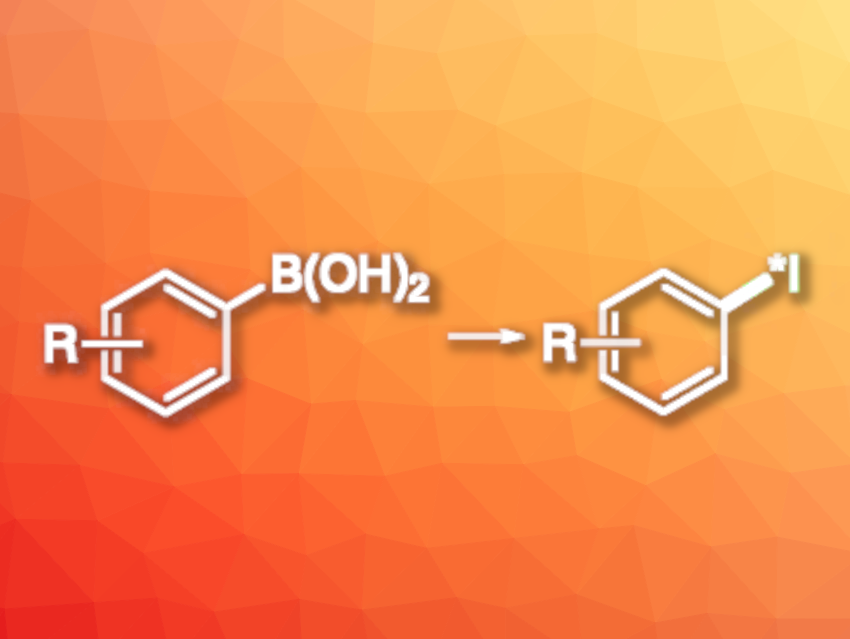
Molecules labeled with radioactive iodine are important in medicinal chemistry and biochemical research. They have applications, e.g., in medical imaging, radiotherapy, or radioassays. Iodine-131 atoms are used in radiopharmaceuticals, and iodine-125 is used in in-vitro biomedical studies. One common approach to the labeling of organic compounds with radioactive iodine is the radioiodination of arenes. Existing methods for this type of transformation can have drawbacks such as a need for toxic reagents, high reaction temperatures, or high catalyst loadings.
Andrew Sutherland, University of Glasgow, UK, and colleagues have developed a method for the efficient radioiodination of arenes via the halodeboronation of aryl boronic acids. The reactions were performed at room temperature using 1–2 mol% loadings of a copper precatalyst and were completed within 10 min. The team used Cu(OAc)(phen)2]OAc as the precatalyst, which was readily prepared from Cu(OAc)2 and 1,10-phenanthroline (phen), [125I]NaI or [123I]NaI as sources of radioactive iodine, and a methanol/water mixture as the solvent.
Under these conditions, a variety of (hetero)aryl and alkenyl boronic acids were converted to the desired radiolabeled products with high radiochemical conversions. The precatalyst was compatible with unprotected amines, which the team used to prepare meta-iodobenzylguanidine ([123I]MIBG), a compound used for the imaging and treatment of tumors. This shows the potential utility of the developed approach.
- Ligand-Enabled Copper-Mediated Radioiodination of Arenes,
Holly McErlain, Matthew J. Andrews, Allan J. B. Watson, Sally L. Pimlott, Andrew Sutherland,
Org. Lett. 2024.
https://doi.org/10.1021/acs.orglett.4c00356