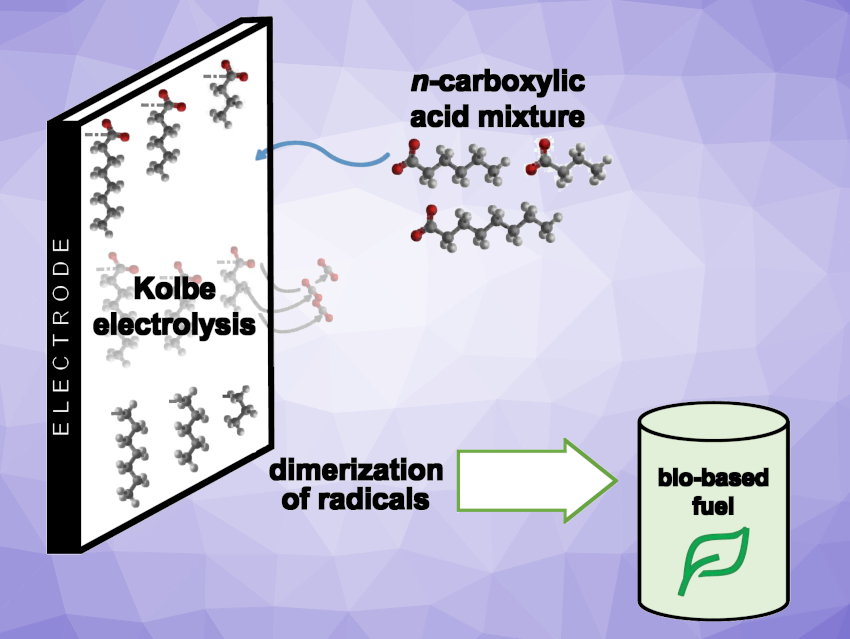
In a more environmentally friendly economy, bio-based resources could replace fossil feedstocks for the synthesis of chemicals and fuels. Electrochemistry is interesting in this context—in particular when renewable electricity is used. For example, the anodic decarboxylation of n-carboxylic acids, also known as Kolbe electrolysis, could be useful in electrobiorefineries.
The Kolbe electrolysis can be performed at ambient temperature and in aqueous solutions, making it environmentally friendly. So far, mainly the Kolbe electrolysis of short-chain n-carboxylic acids at low concentrations has been studied. Medium-chain acids (MCCA) with a chain length between four and eight carbon atoms can be derived from waste and biobased feedstocks using microbial conversions. For an implementation in electrobiorefineries, the conversion of MCCA at higher concentrations is promising, because it gives hydrocarbon mixtures that can be used as fuel additives.
Falk Harnisch, Helmholtz Centre for Environmental Research, Leipzig, Germany, and colleagues have found that electrolyzing a mixture of MCCA at concentrations that resemble the broths gained from bioconversion shows improved performance in comparison to the electrolysis of single acids. The team performed electrochemical conversions of n-butanoic (C4), n-hexanoic (C6), and n-octanoic acid (C8), as well as a mixture of all three, by Kolbe electrolysis
The team found that the preferred reaction pathway was a dimerization with up to 70 % selectivity. However, for a mixture of acids, this type of reaction has to be distinguished between homo- and hetero-coupling. 66 % of the converted hexanoic acid, but only 8–10 % of the converted butanoic and octanoic acid yielded homo-coupled dimers of the resulting radicals. Butanoic and octanoic acid preferably performed hetero-coupling with radicals deriving from hexanoic acid. Thus, hetero-coupling increased the overall conversion significantly when compared with the electrolysis of single acids.
- Hetero‐coupling of bio‐based medium chain carboxylic acids by Kolbe electrolysis enables high fuel yield and efficiency,
Katharina Neubert, Max Hell, Micjel Chávez Morejón, Falk Harnisch,
ChemSusChem 2022.
https://doi.org/10.1002/cssc.202201426



Hallo,
was sagen “Altmeister” Hans Schäfer oder z.B. sein Schüler Andreas Weiper-Idelmann dazu? Bisher war mein Kenntnisstand, dass man Pt-Elektroden braucht, deren Standzeit relativ gering ist. Oder klappt das ganze jetzt mit anderem Elektrodenmaterial, z.b. mit bordotiereten Diamantelektroden?
Gruß
Hermann Pütter
Lieber Herr Pütter,
die Kolbe-Reaktion benötigt leider eine Pt-Oberfläche; allerdings können deutlich günstigere Ti-basierte Pt-beschichtete Elektroden anstelle von Pt-Monolithen zum Einsatz kommen, wie wir in unser vorherigen Studie zeigen konnten:
https://chemistry-europe.onlinelibrary.wiley.com/doi/10.1002/cssc.202100854
Die Stzandzeit muss bzw. wird noch genauer untersucht, allerdings wäre ich da aktuell optimistisch.
Mit bestem Gruss,
Falk Harnisch