Chromium(VI)- and arsenic(V)-based oxyanions are among the most problematic water pollutants due to their high toxicity and prevalence in various water streams. Conventional methods for metal removal often rely on ion exchange and adsorption processes. However, these techniques have limited efficiency, particularly for oxyanions like Cr(VI) and As(V), due to low selectivity and stability.
Jeffrey R. Long, University of California, Berkeley, CA, USA, and colleagues have developed polyol-functionalized porous aromatic frameworks (PAFs) that use targeted interactions such as chelation, redox activity, and hydrogen bonding for selective and high-performance capture of these pollutants.
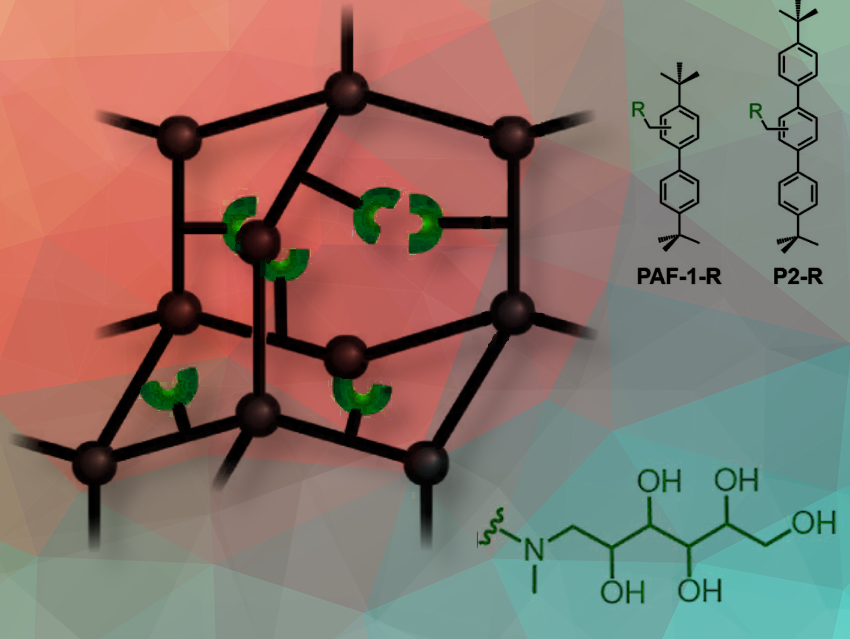
The parent PAF-1 framework consists of tetrahedral carbon nodes connected by biphenyl struts. The team synthesized it through a Yamamoto-type Ullmann coupling reaction starting with the monomer tetrakis(4-bromophenyl)methane. Functional groups (pictured in green) were then added to the framework. A N-methyl-D-glucamine (NMDG) functional group was, for example, appended onto the framework through a facile two-step route, starting with the chloromethylation of PAF-1 before the subsequent nucleophilic addition of NMDG. Other frameworks included (PAF-1-MAPD, PAF-1-serinol, PAF-1-N(CH3)2) containing methylamino-1,2-propanediol (MAPD, featuring a 1,2-diol), serinol (featuring a 1,3-diol), or dimethylamine functional groups, and P2-NMDG.
The researchers found that their newly designed PAFs, especially those functionalized with NMDG, demonstrate rapid adsorption, high capacity, and excellent cycling stability. Adsorption studies reveal that these materials can reach equilibrium within 10 seconds and achieve uptake capacities of 2.5 mmol/g, which are among the highest reported for Cr(VI) and As(V) removal. The unique structure of the PAFs enhances porosity, functional group loading, and the ability to selectively capture these oxyanions, while remaining highly effective even after multiple cycles of use. The material can be recycled using mild acid and base washes without any measurable performance loss over at least ten adsorption–desorption cycles.
Spectroscopic analysis shows that the material’s performance stems from its binding mechanisms, including diol chelation, hydrogen bonding, ion exchange, and redox-interactions via NMDG.
The researchers state that this work establishes key design principles for creating adsorbents for not only chromium and arsenic but also other difficult pollutants like boron and silicon oxyanions, marking a significant advancement in water purification technologies.
- Removal of Chromium and Arsenic from Water Using Polyol-Functionalized Porous Aromatic Frameworks,
Adam A. Uliana, Ethan R. Pezoulas, N. Isaac Zakaria, Arun S. Johnson, Alex Smith, Yubing Lu, Yusuf Shaidu, Ever O. Velasquez, Megan N. Jackson, Monika Blum, Jeffrey B. Neaton, Junko Yano, Jeffrey R. Long,
J. Am. Chem. Soc. 2024.
https://doi.org/10.1021/jacs.4c05728