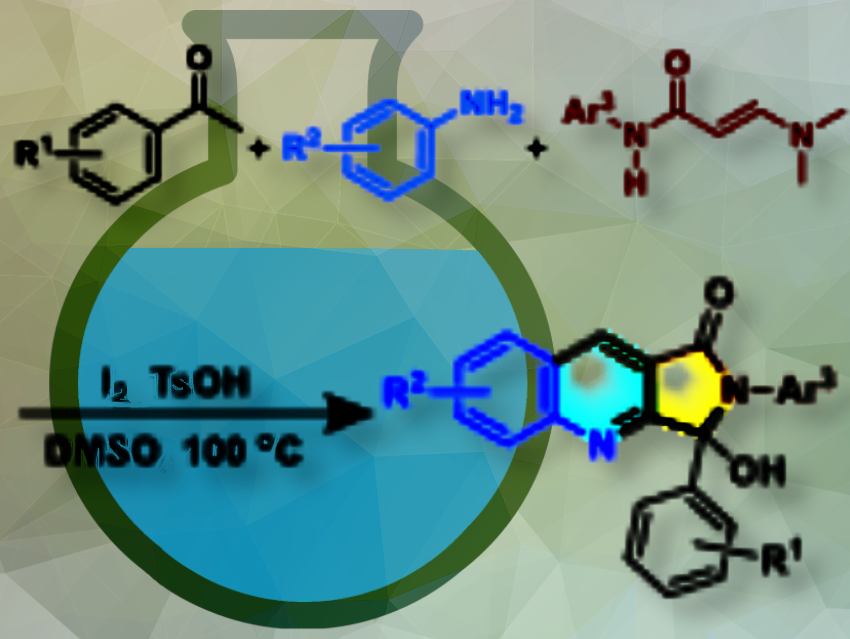
Synthetic compounds with quinoline and pyrrole frameworks are important in drug design due to their presence in many natural alkaloids and their core structure in several active molecules, including promising antibacterial agents and matrix metalloproteinase inhibitors. However, the efficient synthesis of derivatives such as 3-hydroxy-1H-pyrrolo[3,4-b]quinolin-1-one in a one-pot reaction has not been reported so far.
An-Xin Wu, Central China Normal University, Wuhan, and Lanzhou University, both in China, and colleagues have developed a multicomponent cascade cyclization reaction using aryl methyl ketones, aryl amines, and an easily available enaminamide reagents (pictured). This one-pot, metal-free process successfully constructed the 3-hydroxy-1H-pyrrolo[3,4-b]quinolin-1-one skeleton with high substrate compatibility, as they have demonstrated by 50 examples.
The reaction simultaneously formes two C-C bonds, two C-N bonds, and a tetrasubstituted carbon stereocenter with a hydroxyl group. The α’-N and α,β-C sites (C=C) of the enaminamides act as bi-nucleophilic and mono-electrophilic reagents, allowing the efficient construction of the desired skeleton without the need for metal catalysis.
According to the researchers, their method not only simplifies the synthesis of 3-hydroxy-1H-pyrrolo[3,4-b]quinolin-1-one but also opens new avenues for further research into enaminamides and the development of nitrogen-containing fused heterocycles.
The Reaction
The reaction was carried out without inert gas protection. Acetophenone (model substance), iodine, and dimethyl sulfoxide were heated at 100 °C, followed by the addition of p-toluidine (model substance), enaminamide (model substance), and TsOH·H2O at room temperature, and continued heating. The mixture was quenched, extracted, and the product was purified by column chromatography.
The proposed reaction mechanism starts with acetophenone undergoing iodination and Kornblum oxidation to form phenylglyoxal. Phenylglyoxal condenses with p-toluidine to form an imine intermediate, which then undergoes a [4 + 2] cycloaddition with enaminamide. In the presence of TsOH, the resulting intermediate eliminates dimethylamine, undergoes tautomerization, intramolecular nucleophilic cyclization, and finally oxidative aromatization to yield the final product.
- One-Pot Synthesis of 3-Hydroxy-1H-Pyrrolo[3,4-b]quinolin-1-one via Enaminamides,
Xi Shen, Zhi-Cheng Yu, You Zhou, Yandong Wu, Anxin Wu,
Adv. Synth. Catal. 2024.
https://doi.org/10.1002/adsc.202400591