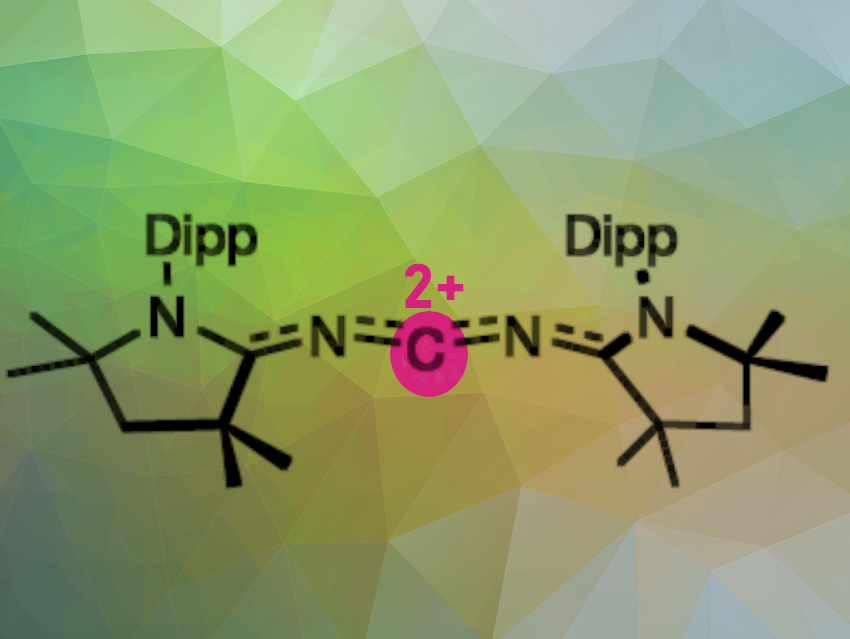
Ying Kai Loh and Guy Bertrand, University of California San Diego, La Jolla, CA, USA, and colleagues have for the first time synthesized and isolated a crystalline doubly oxidized carbene (R2C2+; pictured). The crystalline dication has two counter anions and was prepared by a two-electron oxidation/oxide ion abstraction sequence from the electron-rich bis(imino)carbene.
The team treated bis(imino)carbene with I2 followed by NaOSiMe3 to give a neutral bis(imino)carbonyl compound. Treatment with trifluoric anhydride as an O2-abstracting agent gave the new species. Oxygen temporarily formed a double bond with the two free carbon electrons which allowed the scientists to separate the oxygen along with the two electrons. In an earlier experiment, they tried to remove the binding electrons individually. However, this produced a radical cation that reacted with the solvent.
The result is a carbene molecule in which the central carbon atom has only two bound electrons and two empty electron orbitals. This is a completely new molecular form of carbon—a carbon with only four valence electrons. The molecule shows a zigzag geometry in which the terminal cyclic (alkyl)(amino)carbenes (CAACs) are bent in cis configuration. The lone pairs on the N-atoms are involved in π donation with the central C-atom and π back-bonding with the terminal CAAC carbon atoms. The doubly oxidized carbene is isoelectronic with [4]cumulenes (R2C=C=C=C=CR2), and can be considered its dicationic nitrogen analogue.
This work shows that bulky, strong electron-donor substituents can be used both to mask vacant orbitals at the central carbon atom and to prevent the two anions from coordination. This paves the way for the isolation of a variety of doubly oxidized carbenes, the team says.
- A crystalline doubly oxidized carbene,
Ying Kai Loh, Mohand Melaimi, Milan Gembicky, Dominik Munz, Guy Bertrand,
Nature 2023.
https://doi.org/10.1038/s41586-023-06539-x