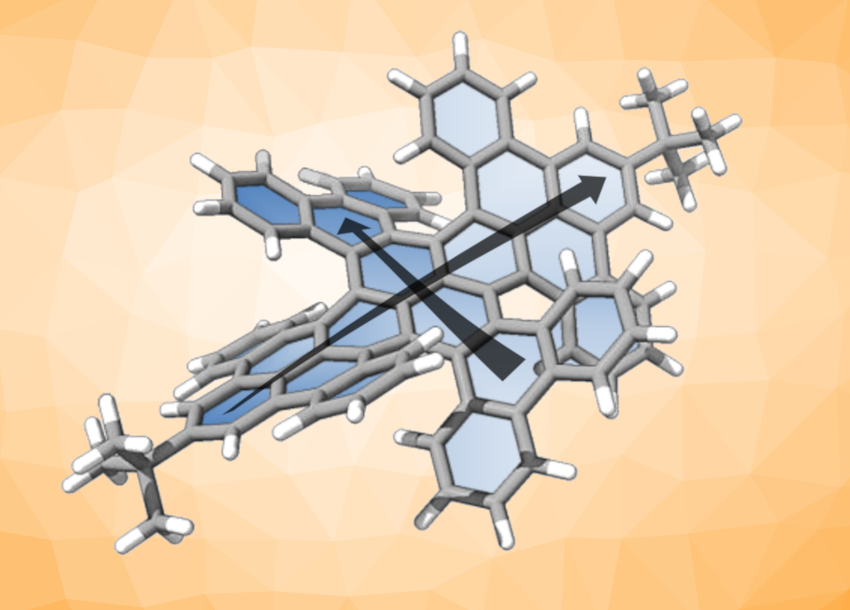
Chiral nanographenes can have interesting structures and useful properties for applications, e.g., in chiral sensors. Often, helicenes are used to prepare chiral nanographenes, for example, by arranging them around an aromatic core. Helicenes are polycyclic aromatic hydrocarbons (PAHs) with a helix-like structure. In some cases, installing helicenes can lead to a twist in the (normally planar) aromatic core of the molecule. The synthesis of such twisted nanographenes, which may have interesting properties, can be challenging.
Yoshifumi Hashikawa, Kyoto University, Japan, Fenghua Bai, Chaolumen, Inner Mongolia University, Hohhot, China, and colleagues have prepared a double-twisted nanographene with a pyrene core (pictured). Pyrene is a—generally planar—PAH consisting of four fused benzene rings. The team first prepared a diphenanthrenopyrene substituted with m-terphenyl groups via a double Suzuki–Miyaura coupling. Then they used a Scholl cyclization with DDQ (2,3-dichloro-5,6-dicyano-p-benzoquinone) to close the remaining rings and obtain the desired double-twisted nanographene, which has four helical units at the periphery surrounding the pyrene core.
The double-twisted nanographene shows a red emission, can undergo redox processes taking up/releasing up to two electrons, and showed chiroptical responses (different interactions with left- and right-circularly polarized light) both in absorption and fluorescence. It can be chemically oxidized to give a radical cation that absorbs in the near-infrared range (NIR-I and NIR-II). According to the researchers, the contorted pyrene core of the nanographene governs its electronic nature. Thus, they point out that introducing literal twists could also lead to new chiral nanographenes with other cores.
- A Double Twisted Nanographene with a Contorted Pyrene Core,
Yanping Dong, Zhiyu Zhang, Yoshifumi Hashikawa, He Meng, Fenghua Bai, Kenichiro Itami, Chaolumen,
Angew. Chem. Int. Ed. 2024.
https://doi.org/10.1002/anie.202406927