Polyfluoroarenes can have unique and useful properties due to the high electronegativity of the fluorine atoms. They can have applications, for example, in advanced materials, pharmaceuticals, or agrochemicals. Often, the synthesis of compounds with polyfluoroaryl units requires the preparation of polyfluoroaryl halides or metal reagents, which are then reacted with a substrate in a second step. Coupling reactions that use polyfluoroarenes directly would be useful to improve the efficiency of such syntheses.
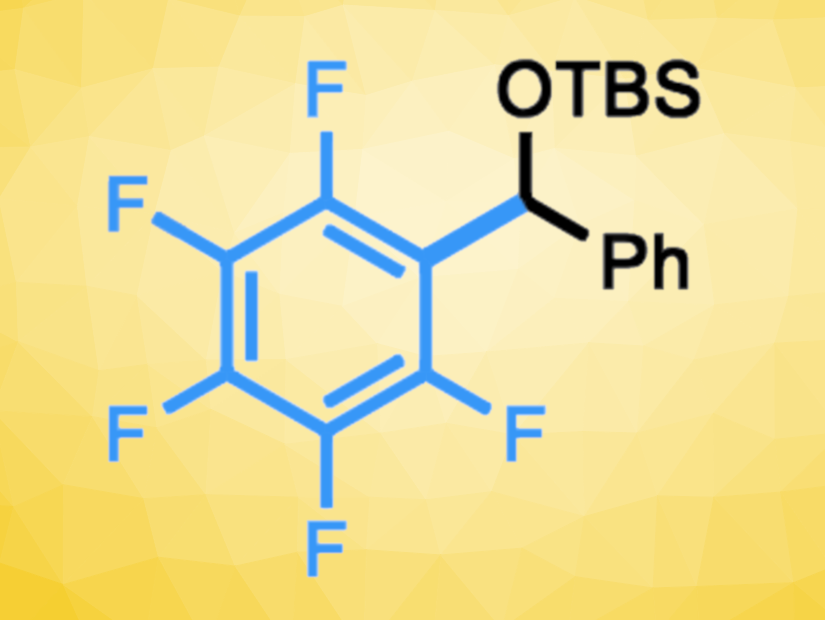
Long Zheng, University of the Chinese Academy of Sciences, Shanghai, Peng Wang, University of the Chinese Academy of Sciences and Hangzhou Normal University, China, and colleagues have developed an approach to the direct coupling of polyfluoroarenes with acylsilanes, leading to t-butyldimethylsilyl (TBS)-protected benzylic alcohols (example product pictured). The reactions proceed under mild conditions and use catalytic amounts of a strong base. The team reacted different polyfluoro(hetero)arenes with a range of acylsilanes of the type R–C(=O)–TBS in the presence of sodium t-amyl oxide as a base. The transformations were performed under a nitrogen atmosphere in dioxane at 30 °C.
The desired coupling products were obtained in mostly moderate to high yields. The researchers conducted a reaction on the gram scale and achieved a yield of 73 %, demonstrating the scalability of the reaction. The products can be desilylated using tetra-n-butylammonium fluoride (TBAF) to obtain the corresponding free alcohols. Overall, the work provides a transition-metal-free path to a wide range of polyfluoroarene and polyfluoroheteroarene derivatives.
- Direct Coupling of Polyfluoroarenes with Acylsilanes Under Metal-Free Conditions,
Muhammad Faisal Yasin, Ying-Chao Li, Long Zheng, Yi-Chen Wu, Peng Wang
Eur. J. Org. Chem. 2025.
https://doi.org/10.1002/ejoc.202500046