Organoboron compounds are becoming increasingly important in materials science and medicinal chemistry, particularly as building blocks in synthetic chemistry, such as in Suzuki-Miyaura and Chan-Lam cross-coupling reactions. While traditional methods such as hydroboration and borylation are well established, there is continued interest in novel C-B bond formation strategies. Recent advances in visible light-induced reactions with diazo compounds have provided mild alternatives for B–H insertion that are key to the synthesis of organoboron compounds. In particular, amine-boranes, with their stability and reactivity, offer promising potential for selective and efficient B–H insertion with minimal side reactions.
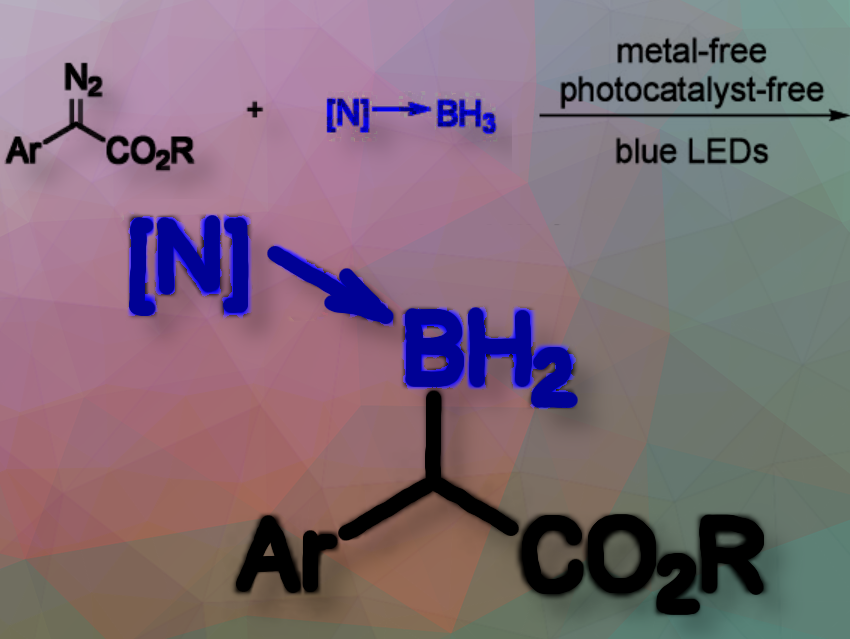
Yan Lu, Zhaoguo Zhang, and colleagues, Shanghai Jiao Tong University, China, have developed a photoinduced, selective, and direct B–H insertion reaction using amine-boranes under mild, metal-free and photocatalyst-free conditions. This approach provides a straightforward route to various organoboron compounds from diazo compounds and amine-borane adducts with moderate to good yields.
The team irradiated a mixture of α-diazo esters and trimethylamine-borane with blue LEDs. The key product, an α-boryl ester, is formed through a B–H bond insertion process. The researchers tested various solvents and conditions, with significant yield improvements achieved by adding molecular sieves to remove water. The reaction works for a wide range of substrates, showing tolerance for different substituents and structures.
The insertion proceeds via a non-radical, concerted pathway where the carbene intermediate generated under light combines with the amine-borane, forming the C–B and C–H bonds simultaneously. The photoinduced formation of a free carbene from α-diazo esters likely is the rate-determining step.
- Visible Light Induced B–H Bond Insertion Reaction with Diazo Compounds,
Mingjun Yi, Xiaoyu Wu, Liqun Yang, Yao YuanYan Lu, Zhaoguo Zhang,
J. Org. Chem. 2024.
https://doi.org/10.1021/acs.joc.4c01510