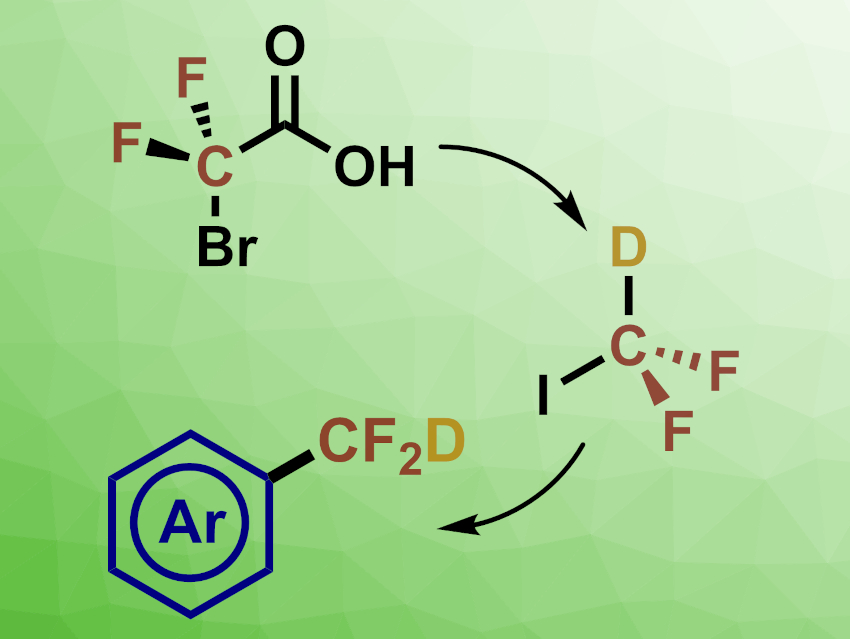
The difluoromethyl group can produce similar biological effects to, e.g., alcohols, amines, or thiols, and can serve as a lipophilic alternative. This makes this functional group interesting for the development of pharmaceuticals and agrochemicals. Ozone-depleting chemicals such as BrCF2H or ClCF2H are often used as reagents to introduce difluoromethyl groups. Difluoroiodomethane (DFIM, ICF2H) could provide similar reactivity with a potentially smaller environmental impact.
Troels Skrydstrup, Aarhus University, Denmark, and colleagues have developed a simple method for the on-demand ex-situ generation of ICF2H and its immediate use in a two-chamber reactor. This allows the team to perform a Pd-catalyzed difluoromethylation of aryl boronic acid derivatives, ultimately giving the corresponding difluoromethylated arene (deuterated version pictured above).
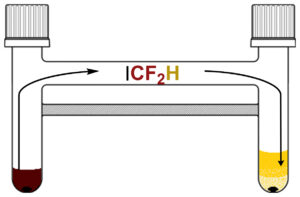 Heating a solution of bromodifluoroacetic acid with sodium iodide in sulfolane generated near-stoichiometric amounts of DFIM. This reactant then moves from the releasing chamber to the reaction chamber of the reactor (pictured on the right), where it takes part in the difluoromethylation of aryl boronic acids and their derivatives. The method can be extended to a two–step difluoromethylation of aryl (pseudo)halides.
Heating a solution of bromodifluoroacetic acid with sodium iodide in sulfolane generated near-stoichiometric amounts of DFIM. This reactant then moves from the releasing chamber to the reaction chamber of the reactor (pictured on the right), where it takes part in the difluoromethylation of aryl boronic acids and their derivatives. The method can be extended to a two–step difluoromethylation of aryl (pseudo)halides.
The also team discovered a simple modification of the conditions in which D2O was added, generating ICF2D. This species is a useful reagent for the installation of –CF2D groups. The researchers propose that this isotopic labeling method could be of high value, e.g., in drug-development programs.
- Pd‐Catalyzed Difluoromethylations of Aryl Boronic Acids, Halides, and Pseudo Halides with Ex Situ Generated ICF2H,
Oliver R. Gedde, Andreas Bonde, Peter I. Golbækdal, Troels Skrydstrup,
Chem. Eur. J. 2022.
https://doi.org/10.1002/chem.202200997