Radicals can be difficult to isolate due to their generally high reactivity. Persistent, stable radicals can be prepared, e.g., when the unpaired electron is delocalized in a cyclic system of π-electrons. There are examples of persistent radicals in organic chemistry, but stable radicals with a purely inorganic cyclic system are rare—and π-delocalized radicals with an entirely inorganic ring had not been isolated so far.
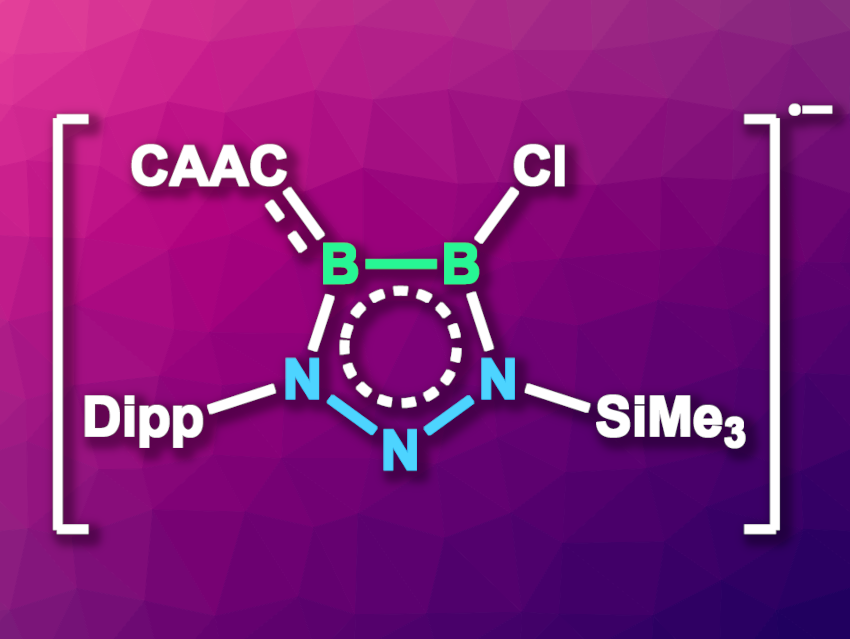
Rei Kinjo, Nanyang Technological University, Singapore, and colleagues have synthesized and isolated a diboratriazole anion radical (pictured, CAAC = a cyclic (alkyl)(amino)carbene, Dipp = 2,6-diisopropylphenyl), in which the unpaired electron is delocalized over the π-electron system. The team prepared the radical anion via a one-electron reduction of the corresponding diboratriazole derivative using potassium graphite (KC8) in benzene at room temperature. The resulting potassium salt, a dark green solid, was recrystallized and then characterized using single-crystal X-ray diffraction. The compound was also studied using electron paramagnetic resonance (EPR) spectroscopy and density functional theory (DFT) calculations.
The researchers found that the radical anion is stable in the solid state and in solution at room temperature, and the results of the DFT calculations indicate that the unpaired electron is delocalized over the five-membered ring and the carbene substituent. The potassium salt of the radical anion can be further transformed into a neutral radical via a reaction with the carbene N,N′-dimethylbenzimidazol-2-ylidene under elimination of KCl. Similarly, a reaction of two equivalents of the radical anion with a biscarbene gives a biradical species in which the biscarbene serves as a linker.
- Crystalline Radical Anion of a Diboratriazole and Its Conversion to a Neutral Radical Driven by a Carbene,
Lizhao Zhu, Zhongtao Feng, Rei Kinjo,
J. Am. Chem. Soc. 2024.
https://doi.org/10.1021/jacs.4c05777