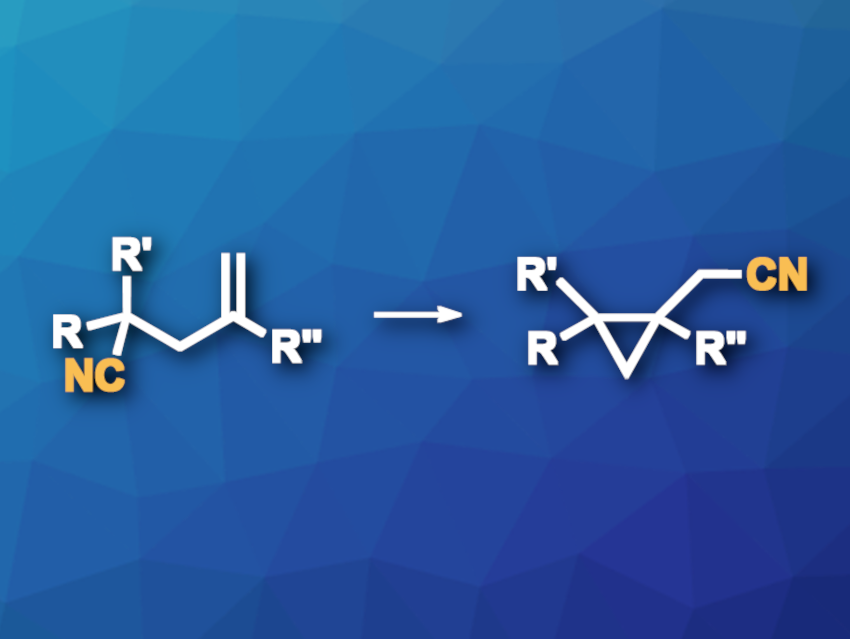
Moving functional groups to another position in a molecule can be a useful type of transformation in organic synthesis. This is often achieved using radical chemistry. The di-π-methane rearrangement is a photochemical reaction involving molecules that contain two π-conjugated systems separated by a one carbon atom, giving cyclopropane derivatives. Transformations of this type can be used to move functional groups, but moving CN groups in these rearrangements had been difficult so far due to an unfavored transition state with a three-membered ring.
Huan-Ming Huang, ShanghaiTech University, China, and colleagues have developed an approach to a di-π-methane-type rearrangement that can be used for CN functional group translocation (general reaction pictured) via a transition state with a five-membered ring. The team used energy-transfer catalysis under visible light to generate a diradical intermediate, which can undergo a 5-exo–dig cyclization to form a five-membered ring with an imine radical, followed by ring-opening and recombination to give the desired product. They used an iridium-based photocatalyst, ethyl acetate (EtOAc) as the solvent, and blue LED light (450 nm). The reactions were performed at room temperature.
Under these conditions, the desired cyclopropane derivatives were obtained in mostly high to excellent yields. The reaction was also used on a gram scale with a yield of 77 %. The reaction has a broad scope and good atom economy.
- Di-π-ethane Rearrangement of Cyano Groups via Energy-Transfer Catalysis,
Yu Zheng, Qi-Xin Dong, Shu-Ya Wen, Hui Ran, Huan-Ming Huang,
J. Am. Chem. Soc. 2024.
https://doi.org/10.1021/jacs.4c04370