1,3-Enynes are useful building blocks in organic synthesis. One atom-economical approach for their preparation is the dimerization of terminal alkynes. Usually, rare and expensive transition metals are used to catalyze those reactions. Replacing those elements with cheaper abundant metals, such as iron, is a current challenge in catalysis research.
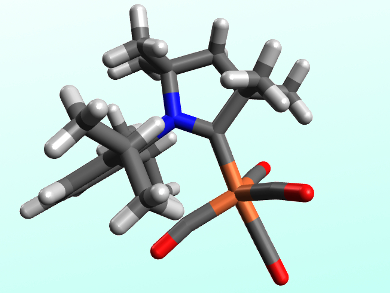
Debashis Adhikari, Swadhin K. Mandal, and colleagues, Indian Institute of Science Education and Research, Kolkata and Mohali, have developed an iron catalyst for the conversion of terminal arylalkynes to the corresponding dimerized enynes. The catalyst, [(cAAC)Fe(CO)4] (pictured), features a cyclic (alkyl)amino carbene (cAAC) ligand, which is similar to the widely used N-heterocyclic carbenes (NHCs) and acts as a strong σ-donor.
The team isolated an alkyne-bound intermediate of the iron complex, which is evidence for the proposed mechanism involving dissociation of one CO ligand, complexation of the first alkyne, ligand exchange of another CO for a deprotonated second alkyne, and the dimerization of the reactants. The researchers were able to prepare a wide range of enynes in good to excellent yields with a catalyst loading of 0.2 mol%. The reactions proceeded with good E:Z selectivity, and the catalyst reached turn-over numbers up to 6500.
- Cyclic (Alkyl)amino Carbene Based Iron Catalyst for Regioselective Dimerization of Terminal Arylalkynes,
Mrinal Bhunia, Sumeet R. Sahoo, Gonela Vijaykumar, Debashis Adhikari, Swadhin K. Mandal,
Organometallics 2016.
DOI: 10.1021/acs.organomet.6b00703