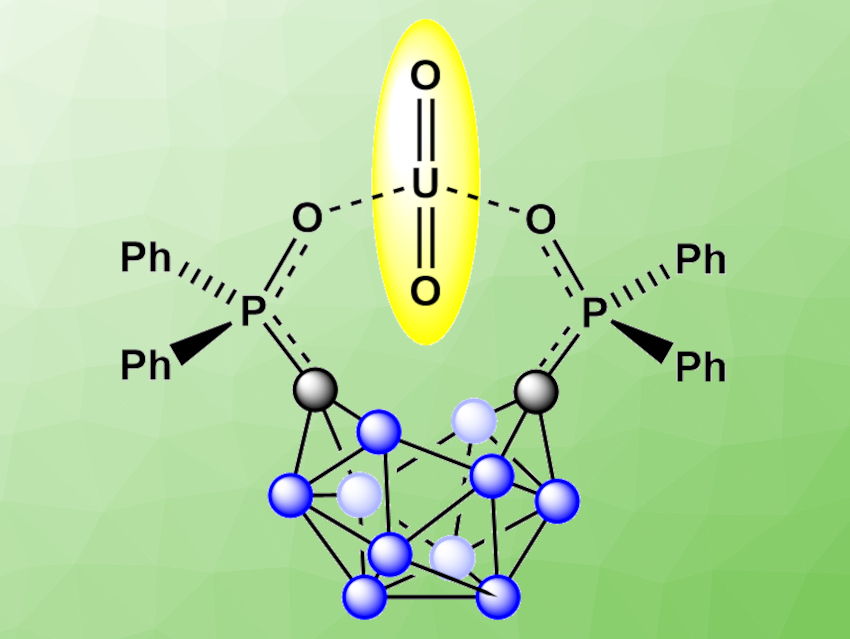
The processing of spent nuclear fuel generally involves the extraction and recycling of uranyl (UO22+) ions. Improved processes for the selective extraction of uranium would be useful to reduce the need for storage space for spent nuclear fuel.
Gabriel Ménard, University of California, Santa Barbara, USA, and colleagues have developed a selective electrochemical biphasic capture and release of UO22+ from aqueous solutions of alkali, lanthanide, and actinide metals that mimic spent nuclear fuel streams, using the ortho-substituted nido-carborane anion [1,2-(Ph2PO)2-1,2-C2B10H10]2− (pictured with a captured uranyl ion). The nido-carborane anion is generated electrochemically from the corresponding closo-carborane by reduction. It was then used to selectively extract uranyl from a mixed-metal aqueous solution into a 1,2-dichloroethane (DCE) organic phase.
After the uranyl capture, the UO22+ was released from the captured product by electrolysis of the DCE phase, which allows the conversion of the nido-carborane anion back to the starting closo-carborane. This process was followed by the extraction of UO22+ into a fresh aqueous phase. The team found that the capture of UO22+ was highly selective.
- Selective electrochemical capture and release of uranyl from aqueous alkali, lanthanide, and actinide mixtures using redox-switchable carboranes,
Megan Keener, Maxwell Mattejat, Shao-Liang Zheng, Guang Wu, Trevor W. Hayton, Gabriel Ménard,
Chem. Sci. 2022.
https://doi.org/10.1039/d1sc07070c