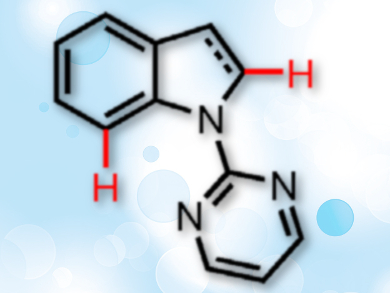
Substituted indolines and indoles are common frameworks in pharmaceutical chemistry. Grignard-type additions of carbonyl compounds are an atom-economical way to introduce substituents to these heterocycles, especially at the C-7 position. A site-selective way of alkylating indoles and indolines using such reactions would be a useful addition to the organic chemist’s toolbox.
In Su Kim, Sungkyunkwan University, Suwon, Republic of Korea, and colleagues have developed such a site-selective Grignard-type C–H addition protocol for coupling N-heterocycles with activated aldehydes and ketones. The team used a ruthenium catalyst to synthesize C-7 alkylated indolines and a rhodium complex to prepare C-2 alkylated indoles (substitution sites pictured).
The team used [Ru(p-cymene)Cl2]2 and AgSbF6 together with sodium acetate to generate the active catalyst. With this approach, they were able to couple a large variety of indolines at the C-7 position to activated carbonyl compounds in dichloroethane solution. A pyrimidyl group on the heterocycle was used as a directing group. Similarly, a catalyst based on [RhCp*Cl2]2, AgSbF6, and NaOAc was used to alkylate a range of pyrimidyl-substituted indoles at C-2 in good to excellent yields. According to the researchers, this work expands the scope of catalytic Grignard-type additions and could be used to generate the scaffolds of biologically active compounds.
- Ruthenium(II)- or Rhodium(III)-Catalyzed Grignard-Type Addition of Indolines and Indoles to Activated Carbonyl Compounds,
Hyeim Jo, Jihye Park, Miji Choi, Satyasheel Sharma, Mijin Jeon, Neeraj Kumar Mishra, Taejoo Jeong, Sangil Han, In Su Kim,
Adv. Synth. Catal. 2016.
DOI: 10.1002/adsc.201600297