The hydrosilylation of alkenes is an important reaction in the production of organosilicon compounds. However, directly using silanes, such as SiH4, MeSiH3, etc., is problematic because they are flammable or even pyrophoric gases at room temperature.
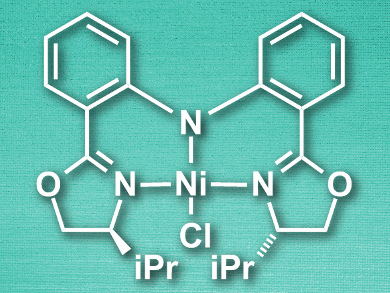
Xile Hu and colleagues, Ecole Polytechnique Fédérale de Lausanne (EPFL), Switzerland, have developed a nickel-catalyzed hydrosilylation reaction that replaces the silanes with alkoxy hydrosilanes, such as Me(EtO)2SiH and (MeO)3SiH. The team used a nickel pincer complex (pictured) as a catalyst and sodium tert-butoxide (NaOtBu) as a base. These conditions allowed the hydrosilylation of a range of terminal alkenes at room temperature with yields up to 93 %.
The researchers propose a mechanism which involves a base-catalyzed disproportionation reaction of the alkoxysilanes to give hydrosilanes, followed by the nickel-catalyzed hydrosilylation of the alkenes. The hydrosilane intermediates were detected using 1H NMR spectroscopy. The reaction could serve as a safer alternative to directly using silanes, has a broad scope and good functional group tolerance.
- Alkoxy Hydrosilanes As Surrogates of Gaseous Silanes for Hydrosilylation of Alkenes,
Ivan Buslov, Sébastien Carlos Keller, Xile Hu,
Org. Lett. 2016.
DOI: 10.1021/acs.orglett.6b00792