Methicillin-resistant Staphylococcus aureus (MRSA) infections are notoriously difficult to treat. This so-called “superbug” is increasingly resistant even to vancomycin, often a drug of last resort, and therefore new classes of antibiotic drugs are urgently required.
Gui-Xin Cai, Cheng-He Zhou, and colleagues, Southwest University, Chongqing, China, have synthesized a series of imidazole naphthalimides and tested them against a panel of bacteria and fungi. Azole-containing drugs are known for their antimicrobial properties – the imidazole-based metronidazole is a well-known example from the clinic. Naphthalimides are important pharmacophores that interfere with protein–DNA interactions, which combats cancer cells and bacteria.
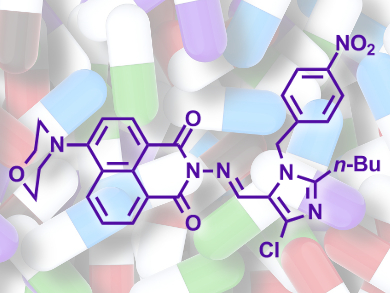
The lead compound in the series, a nitrobenzyl derivative (pictured), inhibits the growth of MRSA and other strains with improved activity over the clinically used chloromycin and norfloxacin. The fast rate of action suggests that permeabilization of the bacterial cell membrane is involved. Preliminary experiments also show that the compound can avoid the bacteria’s efforts to develop drug resistance, which might prove to be an advantageous property for new antibiotics.
- Synthesis and biological evaluation of Schiff base-linked imidazolyl naphthalimides as novel potential anti-MRSA agents,
Huo-Hui Gong, Kishore Baathula, Jing-Song Lv, Guixin Cai, Cheng-He Zhou,
Med. Chem. Commun. 2016.
DOI: 10.1039/C5MD00574D