Homogeneous catalysis using gold complexes has made tremendous progress in recent years. Gold(I) species with neutral ligands are especially effective for the activation of carbon-carbon triple bonds towards nucleophiles. This is due to their soft Lewis-acidic character, a result of the contraction of the gold atom’s 6s orbital by relativistic effects. However, there are few systematic studies on the ligands’ influence on the catalytic activity and stability of different gold (I) complexes.
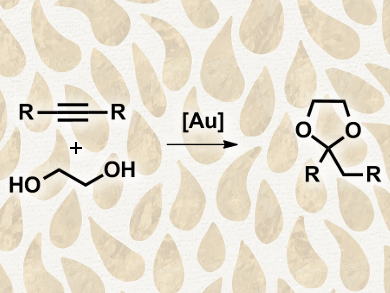
José M. López-de-Luzuriaga and colleagues, Universidad de La Rioja, Spain, studied the activity of different types of [AuL]+ catalysts (L = tertiary phosphines, ylides, or N-heterocyclic carbenes), using the synthesis of cyclic acetals from the alkynes and ethylene glycol as a benchmark reaction. The catalysts were tested in the acetal synthesis in toluene at 100 °C for a duration of four hours under an argon atmosphere. The team found moderate to very low activity when using tertiary phosphines or nonstabilized ylides as ligands, but high catalytic activities for N-heterocyclic carbene complexes.
The researchers used both theoretical and experimental evidence to explain this behavior. Density Functional Theory (DFT) calculations pointed to very similar alkynophilicities for the catalysts regardless of the ligand. The team performed time-resolved NMR spectroscopy to study the stability of the gold(I) complexes during the reaction, and could show that the phosphine- and ylide-based catalysts were transformed into inactive species, such as metallic gold, within 10–50 minutes. Thus, catalyst deactivation is the key reason for the different performance of [AuL]+ catalysts.
- Experimental and Theoretical Study of the Effectiveness and Stability of Gold(I) Catalysts Used in the Synthesis of Cyclic Acetals,
Jesús Cordón, José M. López-de-Luzuriaga, Miguel Monge,
Organometallics 2016.
DOI: 10.1021/acs.organomet.5b01015