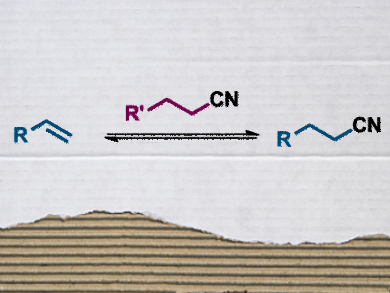
Both nitriles and alkenes are important intermediates in the synthesis of many useful organic compounds. Their easy and controllable interconversion, especially without involving toxic HCN gas, would be a very useful addition to every organic chemist’s toolbox.
Bill Morandi and colleagues, Max Planck Institute for Coal Research, Mülheim an der Ruhr, Germany, have developed a nickel-catalyzed transfer hydrocyanation that adresses this need. The reaction uses aliphatic nitriles, specifically isovaleronitrile, instead of HCN for the hydrocyanation of alkenes (see picture). The driving force of the reaction is the release of a gaseous, stable alkene, which ensures optimal conversion.
The reverse approach can be used for the synthesis of alkenes from nitriles, i.e., retro-hydrocyanation. Here, the reaction is driven by the release of ring strain in the alkene used to accept the CN group. The researchers found that norbornadiene is an efficent trapping agent for this purpose.
The technique was tested for a wide range of substrates, and was shown to be broadly applicable. The reactions proceed in good to excellent yields and with good functional group tolerance. Current limitiations of the method are the hydrocyanation of unprotected alcohols and dienes.
- Catalytic reversible alkene-nitrile interconversion through controllable transfer hydrocyanation,
X. Fang, P. Yu, B. Morandi,
Science 2016, 351, 832–836.
DOI: 10.1126/science.aae0427