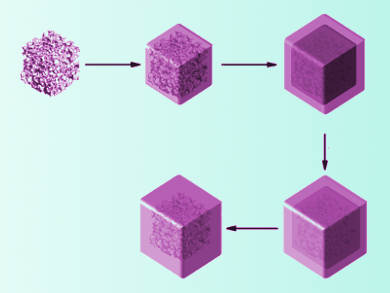
Lithium-ion batteries are a very common rechargeable portable power source. Enhancing their electrochemical performance would be helpful for the storage of renewable energy and other large-scale applications. Nanoparticles composed of SnO2 show very high initial capacities when used as anode materials for these batteries. However, the uptake of lithium ions is not entirely reversible, and the capacity drops significantly with further charge cycles.
Xinhai Li, Central South University, Changsha, China, Guozhong Cao, University of Washington, Seattle, USA, and colleagues have synthesized porous SnO2 submicrocubes (SMCs), which they encapsulated in carbon shells. The SnO2 SMCs were generated by annealing and HNO3 etching of CoSn(OH)6 submicrocubes. Then the cubes were coated with SiO2 and a resorcinol-formaldehyde resin. Carbonization of the resin at 550 °C and removal of the SiO2 layer with hydroflouric acid lead to the final yolk-shell particles. The encapsulated submicrocubes exhibit an increased capacity and cycling stability compared to bare SnO2 nanoparticles when used as an anode material.
The team attributed the improved reversibility of lithium uptake to the porous structure of SnO2 and the space between oxide core and carbon shell, which both act as a buffer for volume expansion upon lithium insertion. The cubes’ porosity also allows the electrolyte solution to easily enter the material, while the carbon shell improves the conductivity of the composite. According to the researchers, the material can be used as a stable and high-energy anode material for lithium-ion batteries.
- Novel Carbon-Encapsulated Porous SnO2 Anode for Lithium-Ion Batteries with Much Improved Cyclic Stability,
Bin Huang, Xinhai Li, Yi Pei, Shuang Li, Xi Cao, Robert C. Massé, Guozhong Cao,
Small 2016.
DOI: 10.1002/smll.201503419