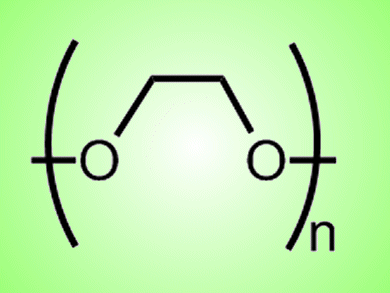
Poly(ethylene glycol) (PEG) is used as a link or an inert spacer to immobilize proteins or peptides on surfaces in range of biotechnological applications. The conformation of PEGylated peptides depend on the local environment, which may be influenced by interaction with the PEG spacer.
Alan Mark and team, University of Queensland, Australia, have performed a molecular dynamics study to determine the extent of the peptide-PEG interaction. Five peptides with differing physical−chemical properties were studied free in solution, attached to a PEG-11 spacer in solution, and constrained to a two-dimensional lattice, mimicking a surface-bound peptide.
PEGylation did not notably affect the conformational properties of neutral or weakly polar peptides. However, highly charged peptides had more extended conformations under low salt conditions due to PEG affecting their solvation properties.
- Effect of Poly(ethylene glycol) (PEG) Spacers on the Conformational Properties of Small Peptides: A Molecular Dynamics Study
Y. Xue, M. L. O’Mara, P. P. T. Surawski, M. Trau, A. E. Mark,
Langmuir, 2010.
DOI: 10.1021/la103800h