Generally, reduction of carboxylic acids requires a stoichiometric amount of hydride reagent and produces an equivalent amount of waste. A more atom-efficient route would be catalytic hydrogenation with H2, but this is difficult under mild conditions.
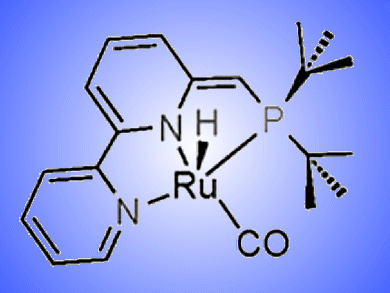
David Milstein and co-workers, Weizmann Institute of Science, Israel, report a dearomatized pyridine-based PNN ruthenium pincer complex that catalyzes the selective and direct hydrogenation of amides to alcohols and amines. The reaction takes place at moderate pressure (10 atm) and under neutral, anhydrous conditions.
The C—N bond is almost exclusively cleaved, with C—O cleavage only observed in trace amounts for anilides. The reaction proceeds in excellent yields, for example, the hydrogenation of N-benzyl-2-methoxyacetamide yielded 2-methoxyethanol (89 %) and benzyl amine (90 %).
- Direct Hydrogenation of Amides to Alcohols and Amines under Mild Conditions
E. Balaraman, B. Gnanaprakasam, L. J. W. Shimon, D. Milstein,
J. Am. Chem. Soc. 2010, 132.
DOI: 10.1021/ja1080019