A frustrated Lewis pair is a compound containing a Lewis acid and a Lewis base that, because of steric hindrance, cannot form an adduct. This makes them chemically rather intriguing species that can react with terminal olefins, alkynes, β-H bonds, disulfides, cyclic ethers, carbon dioxide and nitrous oxide.
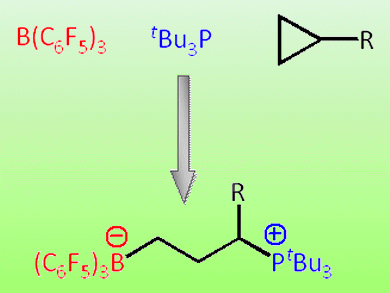
Now, chemists led by Doug Stephen, University of Toronto, Canada, have demonstrated that they can use phosphine/borane frustrated Lewis pairs to convert cyclopropanes into phosphonium borate products through a ring opening reaction. The products could be used as catalysts for many other synthetic reactions without using metals.
- Ring-opening of cyclopropanes by “frustrated Lewis pairs”
J. G. M. Morton, M. A. Dureen, D. W. Stephan,
Chem. Commun. 2010.
DOI: 10.1039/C0CC02862B