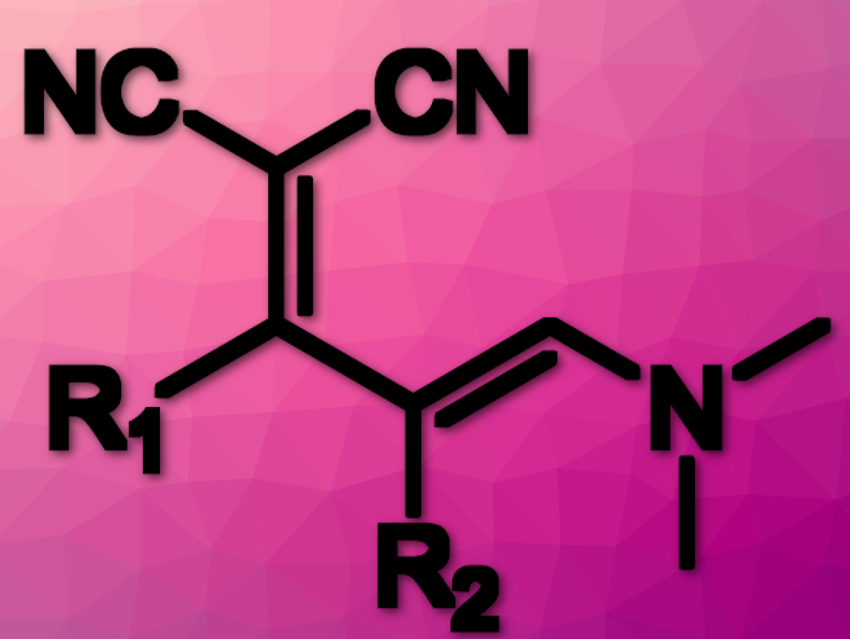
Ylidenemalononitriles (YMs; pictured above) derivatives are intermediates that facilitate the synthesis of multisubstituted heterocycles such as pyridines and pyrones and fluorescent amidine-based substances. However, YMs have a few limitations which prevent them from being used as universal pyridine/pyrone intermediates and their broad use as reactive luminescent probes.
Substituting the R2-position of YMs has several advantages compared to unfunctionalized YMs: an increased range of fluorophores that can be prepared, faster turn-on rates, better color tunability, access to polar-solvent-soluble species, and increased control over cyclization rate. However, functionalizing R2 has remained a challenge.
Kleber T. de Oliveira, Universidade Federal de São Carlos, Brazil, D. Tyler McQuade, Virginia Commonwealth University, Richmond, USA, and colleagues have developed a new approach for functionalizing the R2-position of ylidenemalononitriles (YMs) using strong electrophiles.
A wide range of aryl/alkyl−sulfenyl chloride (RSCl) species reacts with YMs in good to excellent yields resulting in R2-substituted YMs (pictured below). These react with amines to form cyclic amidines. Whereas the starting linear YMs are nonemissive in solution, the cyclic amidines are fluorescent. The researchers observed faster turn-on rates, color tunability, access to polar-solvent-soluble species, and increased control over cyclization rates.

The majority of the cyclic amidines are fluorescent in solution. YMs with electron-deficient or small substituents tended to provide lower yields compared to YMs with other patterns. The emission colors range from green/cyan to yellow. A combination of different R1 and R2 groups yielded different λmax. Large adjacent rings might cause distortions to the ring system raising the highest occupied molecular orbital (HOMO).
Overall, the team says that they have developed a new approach to create turn-on fluorescent materials that have potential applications as bioprobes, sensors, and starting materials for novel light-responsive molecules.
- Increasing Scope of Clickable Fluorophores: Electrophilic Substitution of Ylidenemalononitriles,
Juliana M. de Souza, Irini Abdiaj, Jiaqi Chen, Kenneth Hanson, Kleber T. de Oliveira, D. Tyler McQuade,
J. Org. Chem. 2020.
https://doi.org/10.1021/acs.joc.0c01551