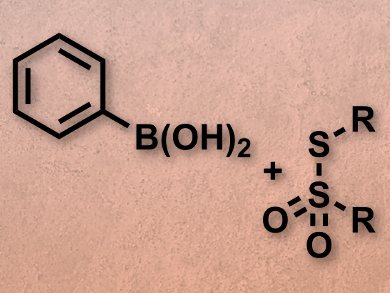Aromatic organosulfur compounds have a variety of uses, e.g., in pharmaceuticals and new materials. Reliable reactions for the formation of C–S bonds at aromatic molecules are relatively rare and often involve the very nasty smell of organosulfur compounds.
Suguru Yoshida, Takamitsu Hosoya, both Tokyo Medical and Dental University, Japan, and colleagues have developed an efficient and odorless pathway to aryl and alkenyl sulfides using organoboron substrates, thiosulfonates as a sulfur source (pictured), and a copper sulfate catalyst. The organoboron compounds were prepared by iridium-catalyzed C–H borylation. The deborylthiolation then afforded a range of aryl and alkenyl sulfides with good yields between 73 % and 98 %.
The reaction allows for easy preparation of previously difficult to access sulfides, and the ready availability of thiosulfonates provides a wide range of possible products.
- A mild and facile synthesis of aryl and alkenyl sulfides via copper-catalyzed deborylthiolation of organoborons with thiosulfonates,
Suguru Yoshida, Yasuyuki Sugimura, Yuki Hazama, Yoshitake Nishiyama, Takahisa Yano, Shigeomi Shimizu, Takamitsu Hosoya,
Chem. Commun. 2015.
DOI: 10.1039/c5cc07463k