An analysis of FDA-approved small-molecule drugs found that 21 % of them contain a saturated 6-membered ring with two heteroatoms, such as nitrogen or oxygen, within their structures. Consequently, new synthetic methods to produce these sorts of heterocycles are sought. Although a number of methods exist to synthesize 6-membered ring structures, such as morpholines and piperazines, and similar 7-membered ring compounds, such as azepines and oxazepines, often the use of expensive or toxic reagents detracts from their applicability in industry.
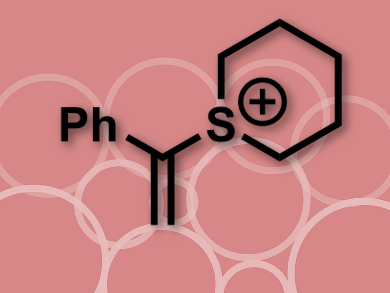
Eoghan McGarrigle, Varinder Aggarwal, and colleagues, University of Bristol, UK, and University College Dublin, Ireland, have developed a concise synthesis for substituted 6- and 7-membered ring systems. By reacting a substituted amino alcohol or diamine with a ring-forming reagent (α-phenylvinylsulfonium salt, cation pictured) and a base, the ring systems are formed with good selectivity and in moderate to excellent yields (27–75 %).
This highly practical route has many advantages that make it suitable for use in industry: reactions are conducted open to the air, solvents are not dried prior to use, and reactions can be performed on the gram scale.
- Synthesis of 6- and 7-Membered N-Heterocycles Using α-Phenylvinylsulfonium Salts,
Johnathan V. Matlock, Thomas D. Svejstrup, Pradip Songara, Sarah Overington, Eoghan M. McGarrigle, Varinder K. Aggarwal,
Org. Lett. 2015.
DOI: 10.1021/acs.orglett.5b02516