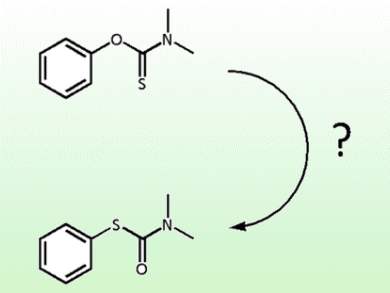
The Newman−Kwart rearrangement (NKR) is one of the prime routes to thiophenols and is generally considered to proceed via an intramolecular migration. Recently J. P. Gilday et al. published a study on the kinetics of the NKR under microwave heating which saw increased conversion at higher concentrations, implying a bimolecular mechanism.
Guy Lloyd-Jones and co-workers, Bristol University, UK, have studied N,N-dimethyl O-p-nitrophenylthiocarbamate in N,N-dimethylacetamide (DMA) under microwave heating for concentrations ranging from 0.11 M to 4.70 M.
Using HPLC, isotope labeling studies and ESI-ICRMS methods, they found the kinetics of rearrangement are exclusively first order at all concentrations studied. No evidence of bimolecularity was seen in crossover experiments. The rise in conversion at higher concentrations seen by Gilday et al. was attributed to higher than expected reaction temperatures due to the setup of the microwave experiment.
- The Molecularity of the Newman−Kwart Rearrangement
M. Burns, G. C. Lloyd-Jones, J. D. Moseley, J. S. Renny,
J. Org. Chem. 2010, 75.
DOI: 10.1021/jo1014382 - The Newman−Kwart Rearrangement: A Microwave Kinetic Study
J. P. Gilday, P. Lenden, J. D. Moseley, B. G. Cox,
J. Org. Chem. 2008, 73, 3130-3134.
DOI: 10.1021/jo702633g