Thioesters are usesed in organic synthesis as intermediates, mild acyl transfer agents, and thiol sources. Nevertheless, the reactions of thioacids have not been widely studied because they are not sufficiently available and the usual synthesis methods involve toxic and unpleasant smelling, gaseous hydrogen sulfide. Also, thioacids as thiols have a strong and repulsive smell.
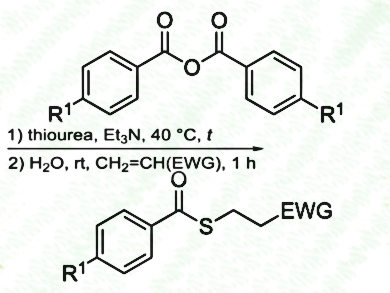
Mohammad Abbasi, Persian Gulf University, Bushehr, Iran, and Reza Khalifeh, Shiraz University of Technology, Iran, have developed an efficient, versatile, and odourless one-pot synthesis of thioesters from non-thiolic precursors under mild conditions. A mixture of a benzoic anhydride, thiourea and an alkyl halide (primary, allylic or benzylic), or a conjugated olefin (ketones, esters, nitriles), Et3N, and H2O produced the related thioesters in good to excellent yields.
- One-pot odourless synthesis of thioesters via in situ generation of thiobenzoic acids using benzoic anhydrides and thiourea,
Mohammad Abbasi, Reza Khalifeh,
Beilstein J. Org. Chem. 2015, 11, 1265–1273.
DOI: 10.3762/bjoc.11.141