Gregory J. Halder and colleagues, Argonne National Laboratory, Argonne, IL, USA, have demonstrated that pressure offers a novel approach for generating new phases and exploring the structure-property relationships of molecular materials.
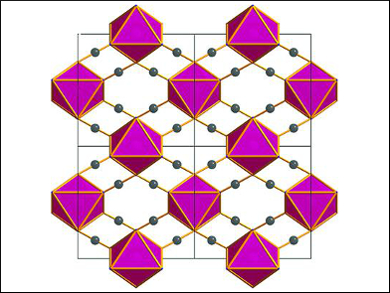
The team used in situ synchrotron powder diffraction to probe the pressure-dependent structural transformation of α-Co(dca)2 [dca = dicyanamide or N(CN)2–] to the high-pressure phase γ-Co(dca)2. This orthorhombic (Pmnn) to monoclinic (P21/n) transformation occurs at 1.1 GPa. The mechanism is driven by a combination of destabilizing factors within α-Co(dca)2, including the elongation of the Co(II) octahedra and the strain on the amide bond angle of the ligand. The reduction in symmetry for γ-Co(dca)2 leads to a tilting effect in the crystal packing, asymmetry in the coordination binding geometries of the cyanide groups of the ligands, and a pronounced rotation of the Co(II) octahedral at high pressures.
Image: Cobalt(II) octahedral packing diagram as viewed in the (001) plane. © Yakovenko et al.
- Pressure-induced structural phase transformation in cobalt(II) dicyanamide,
A. A. Yakovenko, K. W. Chapman, G. J. Halder,
Acta Cryst. 2015, B71, 252–257.
DOI: 10.1107/S2052520615005867