Nitrogen fixation is an important industrial process that converts nitrogen to ammonia, a widely used chemical for fertilizers, explosives, and other products. However, it traditionally requires both high pressure and high temperature since nitrogen is relatively inert. While nature has done this for millions of years under mild conditions, artificial counterparts remain few and far between.
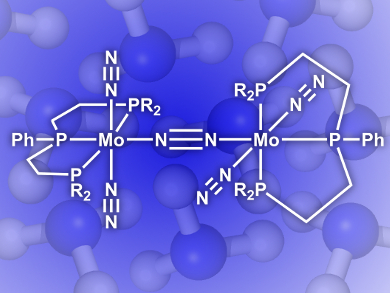
Yoshiaki Nishibayashi, University of Tokyo, Japan, Kazunari Yoshizawa, Kyoto University, Japan, and their colleagues have developed an efficient catalyst that does this job at room temperature and pressure. The nitride-molybdenum complex (pictured) has a triphosphine ligand clamping the Mo center. This unique configuration enables the production of up to 63 molecules of NH3 per Mo, a big leap from the previous record of 26.
As the first successful example of a molybdenum catalyst bearing only one triphosphine ligand that catalyzes direct ammonia formation, this discovery may be further developed into the next generation of nitrogen fixation processes.
- Catalytic Reduction of Dinitrogen to Ammonia by Use of Molybdenum–Nitride Complexes Bearing a Tridentate Triphosphine as Catalysts,
Kazuya Arashiba, Eriko Kinoshita, Shogo Kuriyama, Aya Eizawa, Kazunari Nakajima, Hiromasa Tanaka, Kazunari Yoshizawa, Yoshiaki Nishibayashi,
J. Am. Chem. Soc. 2015.
DOI: 10.1021/jacs.5b02579