Continous-flow synthesis has several advantages over batch synthesis when it comes to reproducibility, automation, using higher pressures and temperatures, and safety. However, the synthesis of complex organic molecules, especially in the pharmaceutical industry, has been a challenge.
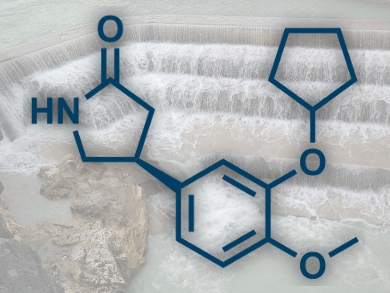
Shū Kobayashi and co-workers, University of Tokyo, Japan, have used a continuous-flow setup to synthesize both enantiomers of rolipram (pictured), a GABA (γ-aminobutyric acid) derivative and anti-inflammatory drug. The team used columns containing both achiral and chiral heterogeneous catalysts, and was able to produce the drug with high enantioselectivity in an eight-step process. Starting from a commercially available aldehyde and nitromethane, they obtained product on the gram scale. Other GABA derivatives could also be synthesized by slightly modifying the system and reactants.
The researchers found their system to be stable for at least a week, and showed that no metal from the employed catalysts found its way into the product. There was no need for isolation of intermediates or for the separation of catalysts, by-products, or excess reagents. The team is working on scaling up their system towards synthesis on a multi-kilogram scale.
- Multistep continuous-flow synthesis of (R)- and (S)-rolipram using heterogeneous catalysts,
Tetsu Tsubogo, Hidekazu Oyamada, Shū Kobayashi,
Nature 2015, 520, 329–332.
DOI: 10.1038/nature14343