Reactive oxygen species (ROS) can cause severe damage in cells, leading to diseases such as cancer. Hence, antioxidants, e.g., phenolic acids and flavonoids, could be very useful for disease prevention. However, their activity is often limited by low solubility in cellular compartments. Lipophilization reactions involve the binding of a lipophilic moiety to the hydrophilic antioxidant. The antioxidant’s solubility in cells is increased by this and it reacts more efficiently with ROS.
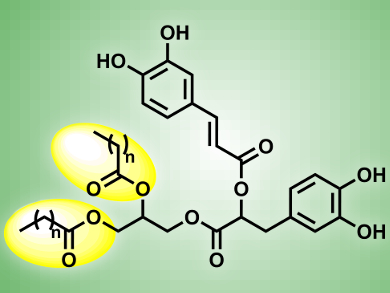
Pierre Villeneuve, University of Montpellier II, France, and colleagues used a two step synthesis to generate 1,2-diacylglycerol rosmarinate (RDAG, pictured): a chemical esterfication based on the Mitsunobu reaction links the antioxidant rosmarinic acid to glycerol, followed by a lipase-catalyzed transesterfication to bind the lipophilic alkyl chains.
Among the synthesized RDAGs with different alkyl chain lengths, RDAG12 (n = 12) shows the best antioxidant activity in cells. Compared to the natural oxidants vitamin E and C, it shows higher ability to reduce ROS levels, making it a promising candidate for further antioxidant research.
- Regioselective synthesis of diacylglycerol rosmarinates and evaluation of their antioxidant activity in fibroblasts,
E. Durand, C. Bayrasy, M. Laguerre, N. Barouh, J. Lecomte, T. Durand, L. Balas, C. Wrutniak-Cabello, G. Cabello, P. Villeneuve,
Eur. J. Lipid Sci. Tech. 2015.
DOI: 10.1002/ejlt.201400607