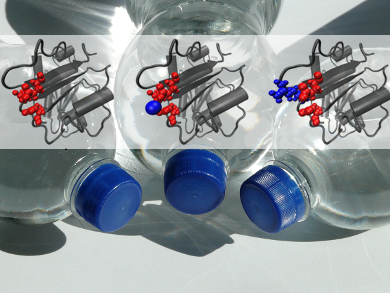
Polyethylene terephthalate (PET) is a synthetic polymer widely used for liquid containers. The worldwide increase in the use of PET is an urgent environmental problem. Since PET is so durable, its recycling and degeneration to monomeric building blocks is highly energy-consuming. Biodegradation by PET hydrolases is a low-energy alternative, but strongly depends on the accessibility of the polymer chains of PET. The accessibility is only sufficient at higher temperatures, where PET becomes more flexible. Unfortunately, the hydrolases are not stable at such elevated temperatures.
Wolfgang Zimmermann and colleagues, Leipzig University, Germany, found a way to avoid this deadlock of mutually exclusive needs: Certain PET hydrolases are more stable at elevated temperatures when divalent cations, such as Mg2+ or Ca2+, are added to the reaction. They identified the binding sites of the cations on the enzyme surface and then replaced negatively charged amino acids with positively charged amino acids by genetic engineering, thus imitating the effect of bound cations. These modified PET hydrolases show an improved thermostability even at 65 °C, making them promising candidates for PET biodegradation.
- Ca2+ and Mg2+ binding site engineering increases the degradation of polyethylene terephthalate films by polyester hydrolases from Thermobifida fusca,
Johannes Then, Ren Wei, Thorsten Oeser, Markus Barth, Matheus R. Belisário-Ferrari, Juliane Schmidt, Wolfgang Zimmermann,
Biotechnol. J. 2015.
DOI: 10.1002/biot.201400620