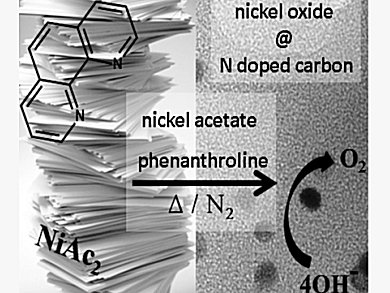
The oxygen evolution reaction (OER) generates molecular oxygen from water. This reaction is a key step for the future development of renewable energy processes and devices, such as direct-solar and electricity-driven water splitting. Therefore, effective catalysts are urgently needed. State-of-the-art catalysts are precious metal catalysts like RuO2 and IrO2. Until now carbon materials have received little attention due to their oxidative sensitivity.
Tim-Patrick Fellinger and colleagues from the Max Planck Institute of Colloids and Interfaces, Potsdam, Germany, have developed a simple, cheap, and scalable synthetic method to produce a nitrogen doped carbon supported nickel catalysts with an enhanced activity. Ordinary cellulose filter paper is soaked into a nickel acetate/phenanthroline solution and carbonized at 800 °C.
This catalyst shows a very good electrocatalytic activity during OER and also high stability with only 5 % loss after 10 h of electrolysis. Additionally, this catalyst can also be used in hydrogen evolution with a good catalytic activity. This enables a symmetric electrolyzer design and makes this cheap catalyst a promising material for wide practical applications.
- Efficient Water Splitting Using a Simple Ni/N/C Paper Electrocatalyst
Jiawen Ren, Markus Antonietti, Tim-Patrick Fellinger
Adv. Energy Mat. 2014.
DOI: 10.1002/aenm.201401660