The control of molecular scale mechanical motions is a highly desirable aim, crucial for understanding many biological processes and for developing biomimetic devices. Of particular interests are molecules capable of unidirectional motion which can yield net work.
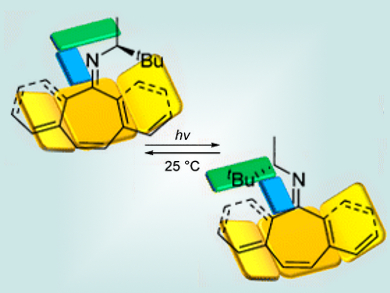
While most such molecular rotors rely on the directional rotation of the C=C bond in overcrowded alkenes, Jean-Marie Lehn and Lutz Greb, Université de Strasbourg, France, have found similar behaviors in imines with steric congestion. These imines can perform two- or four-step unidirectional rotation based on the light- and heat-activated E/Z isomerization of the C=N bond.
Compared to their olefin-based equivalents, the new imine-based molecular rotors are easily accessible through one-step condensation from amines and ketones. In addition, the dynamic exchange reaction unique to imines might lead to other interesting properties in the system.
- Light-Driven Molecular Motors: Imines as Four-Step or Two-Step Unidirectional Rotors,
Lutz Greb, Jean-Marie Lehn,
J. Am. Chem. Soc. 2014.
DOI: 10.1021/ja506034n


![Synthesis of [c2]Daisy Chains via Mechanochemistry](https://www.chemistryviews.org/wp-content/uploads/2025/04/202504_RotaxanesWithSolidStateMechanochemistry-125x94.png)
