Chirality is a key feature in organic chemistry from small molecules to large supramolecular assemblies. Circular dichroism (CD) spectroscopy is used widely to determine the configuration of molecules, which absorb circularly polarized light differently depending on their chirality. However, many small molecules do not give strong CD signals. Consequently, additional probes (either covalently or non-covalently bound) are needed to interact with the small molecules for spectra to be obtained.
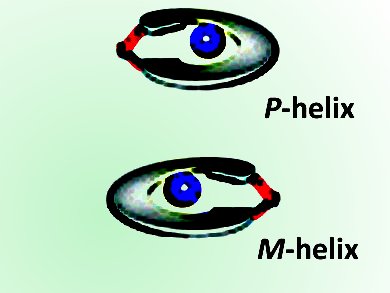
Although a number of such probes exist, Kyu-Sung Jeong and colleagues from Yonsei University, Seoul, Korea, have designed a new dimer probe. The probe contains two aldehyde groups that can react with the two amine groups of chiral 1,2-diamines to form a diimine. The diimine that results folds to either a right-handed or a left-handed helix, and the type of helix formed is directly linked to the configuration of the starting amine molecule.
Consequently, this technique can be used to determine whether compounds are pure or calculate the percentage of chiral forms (enantiomeric excess) within a mixture.
- An indolocarbazole dimer as a new stereodynamic probe for chiral 1,2-diamines,
Hae-Geun Jeon, Min Jun Kim, Kyu-Sung Jeong,
Org. Biomol. Chem. 2014.
DOI: 10.1039/C4OB00872C