Among various ways to reduce CO2 emission, replacing gasoline with cleaner energy sources, such as natural gas, is highly sought-after. However, since natural gas contains 10−20 mol % CO2 when extracted, the removal of CO2 has, therefore, become a prerequisite for effective utilization of natural gas.
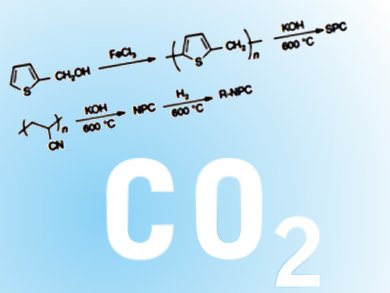
To serve this purpose, James M. Tour at Rice University, Houston, Texas, USA, and colleagues have synthesized sulfur- and nitrogen containing porous carbons from inexpensive thiophene- and acrylonitrile-based polymers, respectively. These nucleophilic materials can chemically adsorb CO2 from natural gas with a capacity up to 82 wt % of sorbent at 30 bar through a hetero atom-initiated CO2 polymerization mechanism.
With excellent selectivity towards CO2, the novel carbon materials require much lower pressure to capture CO2 than existing ones. Furthermore, they can be reinstated upon spontaneous release of CO2 under ambient conditions, without the need for any thermal energy input. All these advantages make them ideal candidates for not only cleaning natural gas, but also CO2 sequestration, among other applications.
- Capturing carbon dioxide as a polymer from natural gas,
C.-C. Hwang, J. J. Tour, C. Kittrell, L. Espinal, L. B. Alemany, J. M. Tour,
Nature Communic. 2014, 5.
DOI: 10.1038/ncomms4961

![Synthesis of [c2]Daisy Chains via Mechanochemistry](https://www.chemistryviews.org/wp-content/uploads/2025/04/202504_RotaxanesWithSolidStateMechanochemistry-125x94.png)

