As an extensively used reaction medium in both research and industrial applications, ionic liquids (ILs) can facilitate a number of reactions, including proton transfer, which is very common in industry. The reactivity of proton transfer is highly dependent on the acidity of the ILs, a property yet to be well characterized.
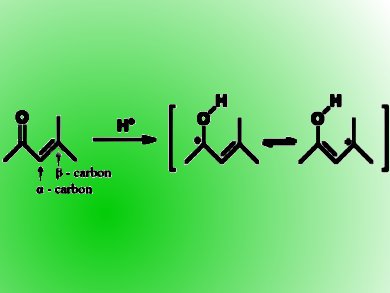
Tom Welton and co-workers, Imperial College London, UK, have reported a convenient method to measure IL acidity between –1 to –9 of the Hammett scale with NMR technique using mesityl oxide as a probe. They relied on the 13C chemical shift difference (∆δ) between the α– and β-carbons of mesityl oxide to extrapolate deduce IL acidity from IL-acid systems.
This approach evades the requirement of colorless samples and the interference of impurities, general problems present in spectrophotometric methods. With a wide measurable acidity range, it also enables quick screening of a large variety of ILs and IL acid solutions.
- A quick, simple, robust method to measure the acidity of ionic liquids,
John Gräsvik, Jason P. Hallett, Trang Quynh To, Tom Welton,
Chem. Commun. 2014.
DOI: 10.1039/C4CC02816C