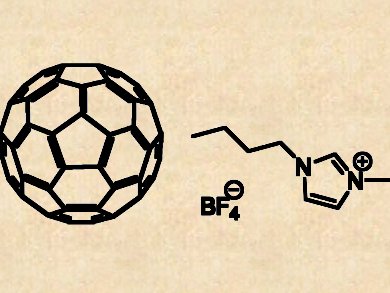
Fullerenes, especially the buckyball C60, prove to be multifaceted in terms of biomedical applications. C60, as a non-polar molecule, is nevertheless insoluble in water, let alone possessing any bioavailability.
Among various strategies to improve the solubility of C60, Eudes E. Fileti of Universidade Federal de São Paulo, SP, Brazil, and Vitaly V. Chaban of Center for Biomembrane Physics, Odense, Denmark, investigate the efficacy of an imidazolium ionic liquid ([C4C1IM][BF4–]) using quantum mechanical calculation.
The seaearchers reveal that the hydrophilic cations in [C4C1IM][BF4–] can form complexes with C60 through π–π interactions, thus effectively separating and dispersing C60 molecules. This approach evades the trouble of tedious covalent chemical modification, and the fact that about 10 to 20 mol% of [C4C1IM][BF4–] is able to completely cover the surface of C60 also minimizes toxicity-associated risks.
- Imidazolium Ionic Liquid Helps to Disperse Fullerenes in Water,
Eudes Eterno Fileti, Vitaly V. Chaban,
J. Phys. Chem. Lett. 2014, 5, 1795−1800.
DOI: 10.1021/jz500609x