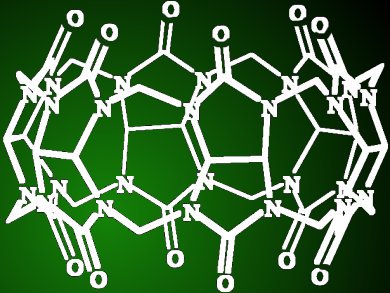
Cucurbiturils are large macrocyclic molecules similar in shape to a pumpkin. They are made up of building blocks (glycoluril units) joined with small bridges (methylene groups), which together form a cavity with oxygen atoms along the rim that point inwards. Although cucurbiturils are well known, their preparation is often challenging.
Frequently, substituent groups need to be added to improve their solubility or allow for further functionalization. Such substituents are generally attached to the building block groups. However, Vladimir Sindelar and colleagues, Masaryk University, Czech Republic, have developed a method in which a single substituent is attached specifically to one of the bridge units. Simple mixing of glycoluril, paraformaldehyde, and another aldehyde formed the substituted cucurbituril. The substituent was confirmed attached to the bridge unit through NMR spectroscopy of X-ray diffractometry studies.
This macrocycle binds organic ammonium cations with similar binding constants as for cucurbituril itself. This new approach will allow access to new substituted cucurbiturils and offers potential for the scope of cucurbituril applications to be broadened.
- Cucurbiturils Substituted on the Methylene Bridge,
Laura Gilberg, Muhammad S. A. Khan, Marketa Enderesova, Vladimir Sindelar,
Org. Lett. 2014.
DOI: 10.1021/ol500828k


![Synthesis of [c2]Daisy Chains via Mechanochemistry](https://www.chemistryviews.org/wp-content/uploads/2025/04/202504_RotaxanesWithSolidStateMechanochemistry-125x94.png)
