Cathleen M. Crudden, Queen’s University, Kingston, ON, Canada, and collegaues from there and from Nagoya University, Japan, have found one of only a few methods available for the enantioselective synthesis of triarylmethanes. Triarylmethanes are an important class of chiral hydrocarbons that have high biological activities and important materials properties.
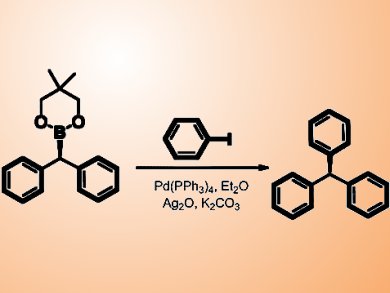
The team carried out the Pd-catalyzed Suzuki−Miyaura coupling of enantioenriched dibenzylic boronic esters with good to excellent stereoretention. The reaction proceeds with almost complete retention of stereochemistry, providing access to triarylmethanes, compounds that have high biological activity and are difficult to prepare in enantiomerically pure form using other methods.
This is the first enantioselective synthesis of dibenzylic boronic esters and contributes to the small but expanding number of stereospecific cross-coupling techniques in the literature.
- Synthesis of Enantiomerically Enriched Triarylmethanes by Enantiospecific Suzuki−Miyaura Cross-Coupling Reactions,
Smitha C. Matthew, Ben W. Glasspoole, Patrick Eisenberger, Cathleen M. Crudden,
J. Am. Chem. Soc. 2014.
DOI: 10.1021/ja412159g¶




Sir, thank you for your work, congratulations.