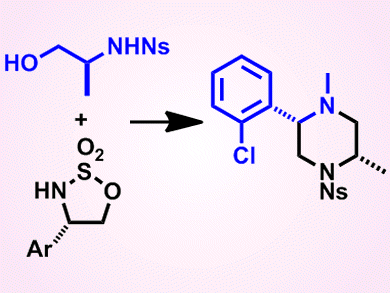
Piperazines, six-membered rings containing two nitrogen atoms in opposite positions, are found in many bioactive molecules, including many drugs. In these active molecules, the piperazine rings are often isolated and the carbon atoms of the ring unsubstituted; however, the potential of fused and/or substituted piperazines as lead-like small molecules is largely unexplored.
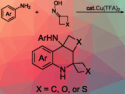
To prepare new lead-like molecules to complement those already present in screening collections, Adam Nelson and colleagues from the University of Leeds, UK, and AstraZeneca, UK, have developed a modular synthetic approach. The approach involves reacting two building blocks, that is, a cyclic sulfamidate and a hydroxy sulfonamide, to produce a range of heterocyclic scaffolds, including piperazines, 1,4-diazepines, and 1,5-diazocanes, in reasonable to good yields. The reaction itself involves ring opening of the cyclic sulfamidate with the hydroxy sulfonamide, followed by cyclization. Importantly, by varying the combinations of building blocks used, it was possible to vary the ring size, substitution, and configuration of the resulting scaffolds, which, after onward reaction, could give new lead-like molecules for use in drug screens.
- A modular lead-oriented synthesis of diverse piperazine, 1,4-diazepane and 1,5-diazocane scaffolds,
Thomas James, Paul MacLellan, George M. Burslem, Iain Simpson, J. Andrew Grant, Stuart Warriner, Visuvanathar Sridharan, Adam Nelson,
Org. Biomol. Chem. 2014.
DOI: 10.1039/C3OB42512F