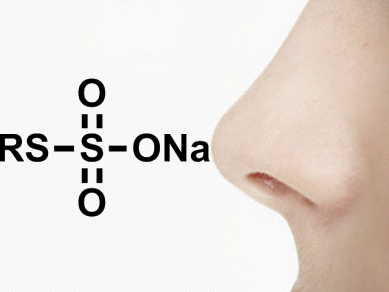
Sulfides are found in many natural and pharmaceutical products, and are often synthetic intermediates for other sulfur-containing molecules. Although a number of methods exist to synthesize sulfides, the starting materials or by-products formed often contain –SH bonds. The drawback is that thiols are notorious for their powerful eggy or garlicky smell. Unsurprisingly, an easy-to-use alternative is welcome.
Jonathan Reeves and colleagues, Boehringer Ingelheim Pharmaceuticals Inc., Ridgefield, CT, USA, have developed a route that avoids thiols to give a nearly odorless approach. This method involves the reaction of the sodium salts of thiosulfuric acid derivatives (RS–S(=O)2O–Na+) with common Grignard reagents (R–MgX; X = halogen) to give sulfides in excellent yields (typically >80 %). The by-product of this mild reaction is an odorless sulfite anion.
This method is general and a broad structural variety of reagents can be used. A selection of Grignard reagents is available commercially or can be generated easily, and derivatives of the easy-to-handle sodium salts (alkyl, aryl and vinyl) can also be made by using standard methods.
- The Reaction of Grignard Reagents with Bunte Salts: A Thiol-Free Synthesis of Sulfides,
Jonathan T. Reeves, Kaddy Camara, Zhengxu S. Han, Yibo Xu, Heewon Lee, Carl A. Busacca, Chris H. Senanayake,
Org. Lett. 2014.
DOI: 10.1021/ol500067f