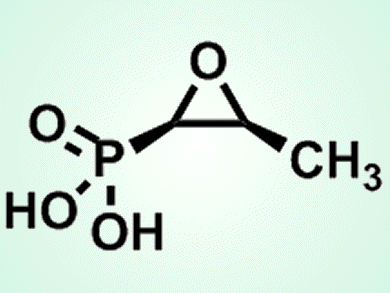
Fosfomycin ((1R,2S)-epoxypropylphosphonate, pictured) is an antibiotic used to treat gastrointestinal and urinary infections. Its pharmacological function depends on its epoxide ring. In bacteria, this group is formed in a reaction catalyzed by S-HPP epoxidase (HppE), an iron-dependent enzyme that converts (S)-2-hydroxypropyl-1-phosphonate (S-HPP) into fosfomycin. This conversion is thought to require the transfer of two electrons and two hydrogen atoms from S-HPP and the concomitant reduction of O2 to two water molecules, in an NAD(P)H dependent reaction.
According to Cheng Wang, Pennsylvania State University, State College, PA, USA, and colleagues this reaction mechanism is not correct. The researchers demonstrated that HppE´s preferred substrate is H2O2 and revealed that HppE abstracts hydrogen atoms from S-HPP not via a O2-derived iron complex, as originally believed, but through a multistep mechanism that involves an FeIV species and a carbocation intermediate. Based on this evidence HppE is not an oxidase but rather a peroxidase.
- Evidence that the Fosfomycin-Producing Epoxidase, HppE, Is a Non-Heme-Iron Peroxidase,
C. Wang, W.-c. Chang, Y. Guo, H. Huang, S. C. Peck, M. E. Pandelia, G.-m. Lin, H.-w. Liu, C. Krebs, J. M. Bollinger,
Science 2013, 342, 991–995.
DOI: 10.1126/science.1240373