Aryl ether motifs are found in many natural products and drugs. Consequently, arylation and alkylation reactions are important transformations in synthesis. Although a number of protocols exist – including copper- or palladium-catalyzed couplings with arylboronic acids – many require expensive catalysts, or high temperatures and long reaction times. Metal-free versions of these reactions also exist but often use toxic reagents or have limited scope.
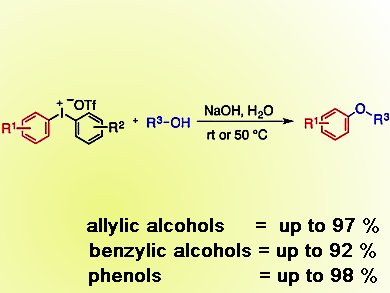
Berit Olofsson and colleagues, Stockholm University, Sweden, have developed a general and simple arylation reaction that uses an iodine-containing salt as reagent. These salts, along with sodium hydroxide in water, form a broad range of aryl ethers by reaction with allylic and benzylic alcohols in good yields. Diaryl ethers also form in excellent yields, starting from phenols.
The salt used, a diaryliodonium salt, contains stable hypervalent iodine and means that this method is metal-free and environmentally benign. In addition, the spent reagent, an iodoarene, was fairly readily recovered and used to reform the starting salt.
Aliphatic alcohols performed poorly, so work in this area to improve methods is expected in due course.
- Metal-Free Synthesis of Aryl Ethers in Water,
Erik Lindstedt, Raju Ghosh, Berit Olofsson,
Org. Lett. 2013.
DOI: 10.1021/ol402960f



![Synthesis of [c2]Daisy Chains via Mechanochemistry](https://www.chemistryviews.org/wp-content/uploads/2025/04/202504_RotaxanesWithSolidStateMechanochemistry-125x94.png)