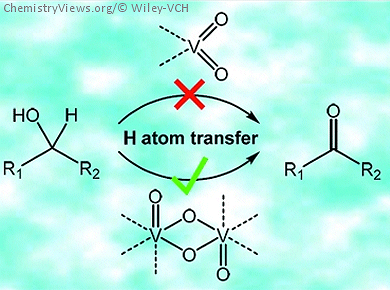
Supported vanadium oxide catalysts are among the most efficient systems for the oxidative dehydrogenation (ODH) of alkanes and alcohols. The reaction mechanisms involving these catalysts have been elucidated by mimicking their postulated active sites on a molecular level. This was achieved by using model compounds with either similar structural and the spectroscopic properties or similar reactivity to corresponding supported vanadium oxide species.
Christian Limberg, Humboldt-Universität zu Berlin, Germany, and colleagues present a detailed mechanistic investigation of the ODH of alcohols catalyzed by a thiobisphenolate-based oxidovanadium model system [1]. In solution, the model system is in an equilibrium between the dimeric and a monomeric form. The results of the kinetic investigations suggested that only the dimer is catalytically active in the ODH of alcohols.
To obtain a deeper understanding of the possible origin of the difference in the ODH activity between monomeric and dimeric oxidovanadium species, they synthesized a strictly monomeric dioxidovanadium complex. Investigation of this monomeric complex as a catalyst for the oxidative dehydrogenation of 9-fluorenol, confirmed its much lower reactivity in comparison to dimeric complexes.
- Direct Proof for a Lower Reactivity of Monomeric vs. Dimeric Oxidovanadium Complexes in Alcohol Oxidation,
C. Gunnar Werncke, Christian Limberg, Ramona Metzinger,
Z. anorg. allg. Chem. 2013, 639, 2426–2432.
DOI: 10.1002/zaac.201300439
[1] C. G. Werncke, C. Limberg, C. Knispel, S. Mebs, Chem. Eur. J. 2011, 17, 12129–12135. DOI: 10.1002/chem.201101442