Initially, host-guest chemistry focused on the encapsulation of small molecules or ions. Recent research designs larger hosts that are capable of encapsulating inorganic catalysts, larger substrates, and even small peptide sequences. Hosts that absorb visible light and exhibit redox chemistry relevant for host−guest photosensitization are interesting for the production of solar fuels.
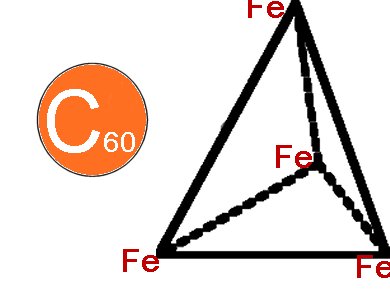
Kingsuk Mahata, Peter D. Frischmann, and Frank Würthner, Universität Würzburg, Germany, have designed, isolated, and studied the host−guest chemistry of new metallosupramolecular tetrahedra Tn. It is formed by self-assembly of octahedral Fe(II) ions and linear perylene bisimide (PBI) dyes with 2,2′-bipyridine groups covalently attached at the imide positions. The Fe4(PBI)6 tetrahedron consists of photo- and redox-active PBI edges and [Fe-(bpy)3]2+ corners. With a diameter of roughly 3.9 nm they are among the largest metallosupramolecular tetrahedra reported. The encapsulation of C60 proves that the tetrahedra is an excellent host for large guest molecules.
The tetrahedron absorbs strongly across the visible spectrum out to 650 nm. It exhibits a total of seven highly reversible electrochemical oxidation and reduction waves spanning a 3.0 V range. This facile cycling of 34 electrons between +18 and −16 charged species is likely enabled due to the porous nature of the tetrahedron. This allows the necessary counterions to freely flow in and out of the host.
Future investigations are focused on the application of these multifunctional tetrahedra as sensitizers for the photo- or electrocatalytic conversion of encapsulated guests.
- Giant Electroactive M4L6 Tetrahedral Host Self-Assembled with Fe(II) Vertices and Perylene Bisimide Dye Edges,
Kingsuk Mahata, Peter D. Frischmann, Frank Würthner,
J. Am. Chem. Soc. 2013.
DOI: 10.1021/ja4083039


![Synthesis of [c2]Daisy Chains via Mechanochemistry](https://www.chemistryviews.org/wp-content/uploads/2025/04/202504_RotaxanesWithSolidStateMechanochemistry-125x94.png)
