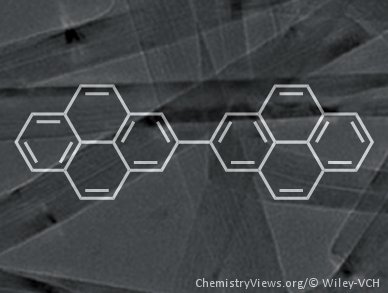
Polycyclic aromatic hydrocarbons (PAHs) have many applications as chromophores, mesogens, and semiconductors but the precise synthesis of extended analogues is difficult.
Klaus Müllen of the Max-Planck-Institut for Polymer Research, Mainz, Germany, and colleagues have previously used the cyclodehydrogenation of non-planar oligophenyl and oligoaryl precursors to construct giant PAHs. Now, they have fused bi- and terpyrenyls selectively to make other extended PAHs without using harsh conditions. This is the first demonstration of how pyrene units can be used as valid precursor molecules for the synthesis of defined extended PAHs despite the need to form an otherwise difficult to synthesize 4,4′ carbon–carbon bond.
The team explains that the pyrenes have advantages over benzene-type precursors that could allow them to make ribbons of graphene-like molecules.
- The Right Way to Self-fuse Bi- and Terpyrenyls to Afford Graphenic Cutouts,
Dominik Lorbach, Manfred Wagner, Martin Baumgarten, K Müllen,
Chem. Comm. 2013.
DOI: 10.1039/C3CC45235B